NIKKOMYCIN Z
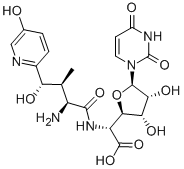
- CAS No.
- 59456-70-1
- Chemical Name:
- NIKKOMYCIN Z
- Synonyms
- NIKKOMYCIN;huaguamycin;neopolyoxinc;NIKKOMYCIN Z;(2s-(2r*,3r*,4r*))-l);NIKKOMYCIN Z, STREPTOMYCES TENDAE;Nikkomycin Z, Sterptomyces tendae;nikkomycin Z from streptomyces tendae;5-((2-amino-4-hydroxy-4-(5-hydroxy-2-pyridinyl)-3-beta-d-allofuranuronicaci;5-((2-Amino-4-hydroxy-4-(5-hydroxy-2-pyridinyl)-3-beta-d-allofuranuronicacid
- CBNumber:
- CB0767000
- Molecular Formula:
- C20H25N5O10
- Molecular Weight:
- 495.44
- MOL File:
- 59456-70-1.mol
- Modify Date:
- 2024/7/2 8:54:58
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H335-H315-H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| RTECS | BA2928200 |
| F | 10 |
NIKKOMYCIN Z Chemical Properties,Uses,Production
Description
Nucleoside-type antibiotic isolated from culture filtrates of Streptomyces tendae (19). Some analogs were also prepared by mutasynthesis, utilizing a uracil auxotroph of S. tendae.
Uses
Nikkomycins inhibit the growth of various plant pathogenic fungi but are inactive against bacteria and yeasts. Too susceptible to photodegradation by sunlight to be used in field application, and thus not developed commercially for agricultural use. Among the nikkomycins, nikkomycin Z is an orally active antifungal therapeutic agent for coccidioidomycosis. It is a competitive chitin synthase inhibitor that has been evaluated in mouse models for coccidioidomycosis.
Definition
ChEBI: A uridine-based nucleoside-peptide antibiotic which inhibits fungal chitin biosynthesis by inhibiting chitin synthase.
Pharmacology
By inhibiting chitin synthetase in fungi, nikkomycins inhibit cell wall synthesis, ultimately causing fungal cells to swell and burst.
Metabolism
There are no detailed reports on metabolism/transformation of nikkomycins in the environment.
NIKKOMYCIN Z Preparation Products And Raw materials
Raw materials
Preparation Products
NIKKOMYCIN Z Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15416 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-8746-5837 +8619945049750 | China | 9642 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49734 | 58 | Inquiry |
| TargetMol Chemicals Inc. | United States | 38642 | 58 | Inquiry | |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8618501500038 | China | 26362 | 58 | Inquiry |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +8618791163155 | China | 3558 | 58 | Inquiry |
| Aladdin Scientific | United States | 52924 | 58 | Inquiry | |
| RD International Technology Co., Limited | 18024082417 | China | 9835 | 58 | Inquiry |
| MedChemExpress | 021-58955995 | United States | 6398 | 58 | Inquiry |
| Nantong Zhonghe Chemical New Materials Co., Ltd | 13003551299 15950816326 | China | 9173 | 58 | Inquiry |
Related articles
- Mechanism of action of Nikkomycin Z
- Nikkomycin Z is an experimental compound with antifungal properties. Like all nikkomycins, it consists of a pyrimidine nucleos....
- Mar 31,2022





