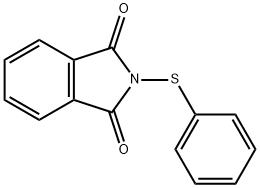
N-(PHENYLTHIO)PHTHALIMIDE
- CAS No.
- 14204-27-4
- Chemical Name:
- N-(PHENYLTHIO)PHTHALIMIDE
- Synonyms
- N-(PHENYLTHIO)PHTHALIMIDE;N-(Phenylthio)phthalimide>N-(Phenylthio)phthalimide;2-(Phenylthio)isoindoline-1,3-dione;2-(Phenylthio)-1,3-isoindolinedione;2-(Phenylthio)-1H-isoindol-1,3(2H)-dione;2-(Phenylthio)-1H-isoindole-1,3(2H)-dione;1H-Isoindole-1,3(2H)-dione, 2-(phenylthio)-
- CBNumber:
- CB1240906
- Molecular Formula:
- C14H9NO2S
- Molecular Weight:
- 255.29
- MOL File:
- 14204-27-4.mol
- Modify Date:
- 2023/4/23 13:52:06
| Melting point | 160-163 °C(lit.) |
|---|---|
| Boiling point | 432.2±28.0 °C(Predicted) |
| Density | 1.42±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO: soluble25mg/mL, clear, yellow |
| pka | -3.05±0.20(Predicted) |
| form | powder to crystal |
| color | White to Light yellow |
| CAS DataBase Reference | 14204-27-4 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Safety Statements | 24/25 |
| WGK Germany | 3 |
| HS Code | 29309090 |
N-(PHENYLTHIO)PHTHALIMIDE price More Price(2)
N-(PHENYLTHIO)PHTHALIMIDE Chemical Properties,Uses,Production
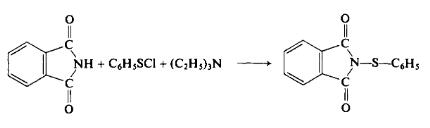
Preparation
(a) Preparation of benzenesulfenyl chloride. To a stirred solution of 55 gm (0.5 mole) of benzene thiol in 300 ml of η -pentane at 0°C is added chlorine gas until an assay (GC) of the resulting red-orange solution shows quantitative conversion to the sulfenyl chloride. The reaction usually requires about 39 gm (0.6 mole) of chlorine.
(b) Reaction of benzenesulfenyl chloride with phthalimide. To a stirred solution of 73.5 gm (0.5 mole) of phthalimide in 200 ml of dimethylforma-mide is first added 60 gm (0.6 mole) of triethylamine. Then the sulfenyl chloride solution from (a) is slowly added dropwise. The reaction mixture is stirred for \ hr, poured into 2 liters of cold water, filtered, and dried to afford 121 gm (95%), m.p. 160-161°C (recrystallized from ethanol).
Complex imides such as camphorimide and 9,10-dihydroanthracene-9,10-endo-a,/3-succinimide have been alkylated in methylene dichloride with alkyl halides, using tetrabutylammonium bromide as a phase-transfer catalyst and aqueous potassium hydroxide as a base.
N-(PHENYLTHIO)PHTHALIMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | China | 35451 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49999 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43348 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |
| Shanghai Boer Chemical Reagent Co., LTD | 19102157732 | China | 7855 | 58 | Inquiry |





