ChemicalBook > Product Catalog >Analytical Chemistry >Standard >Pharmaceutical Impurity Reference Standards >Rosuvastatin EP impurity H
Rosuvastatin EP impurity H
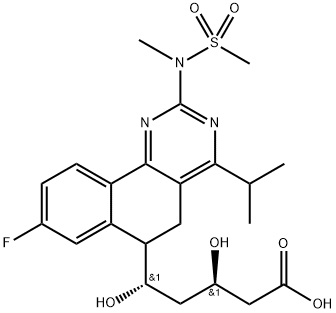
- CAS No.
- 1335110-44-5
- Chemical Name:
- Rosuvastatin EP impurity H
- Synonyms
- ppRSV;Rosuvastatin EP Imp H;Rosuvastatin Impurity QR;Rosuvastatin Impurity 195;Rosuvastatin EP impurity H;Rosuvastatin calcium EP Impurity H;Rosuvastatin Impurity H Sodium Salt;Rosuvastatin EP Impurity H Sodium Salt;Rosuvastation Impurity H (Calcium salt);Rosuvastatin Calcium EP Impurity H (Calcium Salt)
- CBNumber:
- CB12737316
- Molecular Formula:
- C22H28FN3O6S
- Molecular Weight:
- 481.54
- MOL File:
- 1335110-44-5.mol
- Modify Date:
- 2023/5/15 10:43:58
Rosuvastatin EP impurity H Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 43)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SynThink RESEARCH CHEMICALS | +91-8177860948 +91-8177860948 | Mumbai, India | 494 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | China | 11927 | 58 | Inquiry |
| ShenZhen H&D Pharmaceutical Technology Co., LTD | +86-0755-22677845 +86-13627253706 | China | 3389 | 58 | Inquiry |
| Guangzhou PI PI Biotech Inc | 020-81716320 +86-13602409664 | China | 3635 | 55 | Inquiry |
| Hubei De'ao Chemical Research Pharmaceutical Technology Co., Ltd | 18502726233; 18502726233 | China | 9805 | 58 | Inquiry |
| BOC Sciences | 1-631-485-4226; 16314854226 | United States | 14059 | 65 | Inquiry |
| Molcoo Chemicals Inc. | 17386083646 | China | 9243 | 58 | Inquiry |
1335110-44-5(Rosuvastatin EP impurity H)Related Search:
Rosuvastatin EP Impurity E (Calcium Salt)
Pitavastatin Impurity 8
Rosuvastatin Impurity 14
(3R,5R)-Rosuvastatin
N-DESMETHYL ROSUVASTATIN-D3
Rosuvastatin
(3S,5R)-Rosuvastatin Methyl Ester
Rosuvastatin IMpurity (5-Oxo Rosuvastatin tert-Butyl Ester)
Rosuvastatin Anhydro Lactone
Rosuvastatin IsoaMy Ester
Rosuvastatin EP impurity A
(3S,5R)-Rosuvastatin
N-[4-(4-Fluorophenyl)-6-(1-methylethyl)-5-[(1E)-2-[(2S,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethenyl]-2-pyrimidinyl]-N-methylmethanesulfonamide
Rosuvastatin methyl ester
tert-Butyl 6-[(1E)-2-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetate
chevron_left
1of4
chevron_right
Rosuvastatin EP impurity H
(3R,5RS)-5-[8-uoro-2-[methyl(methylsulfonyl)amino]-4-(propan-2-yl)-5,6-
dihydrobenzo[h]quinazolin-6-yl]-3,5-dihydroxypentanoic acid
Rosuvastatin calcium EP Impurity H
Rosuvastatin Impurity H Sodium Salt (Mixture of Diastereomers)
(3R,5RS)-5-[8-Fluoro-4-isopropyl-2-(N-methylmethylsulfonamido)-5,6-dihydrobenzo[h]quinazolin-6-yl]-3,5-dihydroxypentanoicacid calcium salt
Rosuvastatin Impurity QR
ppRSV
Rosuvastation Impurity H (Calcium salt)
Rosuvastatin Impurity H Sodium Salt
Rosuvastatin Calcium EP Impurity H (Calcium Salt)
Rosuvastatin EP Imp H
Rosuvastatin Impurity 2(Rosuvastatin EP Impurity H)
Rosuvastatin EP Impurity H Sodium Salt
Benzo[h]quinazoline-6-pentanoic acid, 8-fluoro-5,6-dihydro-β,δ-dihydroxy-4-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-, (βR,δS)-
(βR,δS)-8-Fluoro-5,6-dihydro-β,δ-dihydroxy-4-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-benzo[h]quinazoline-6-pentanoic Acid
Rosuvastatin Impurity 10(Mixture of Diastereomers)
Rosuvastatin Impurity H Calcium Salt (Mixture of Diastereomers)
Benzo[h]quinazoline-6-pentanoic acid, 8-fluoro-5,6-dihydro-β,δ-dihydroxy-4-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-, (βR,δS)-
(3R,5S)-5-(8-fluoro-4-isopropyl-2-(N-methylmethylsulfonamido)-5,6-dihydrobenzo[h]quinazolin-6-yl)-3,5-dihydroxypentanoic acid? (Rosuvastatin Impurity)
Rosuvastatin Impurity 82 Sodium Salt (Mixture of Diastereomers)
Rosuvastatin Impurity 195
1335110-44-5





