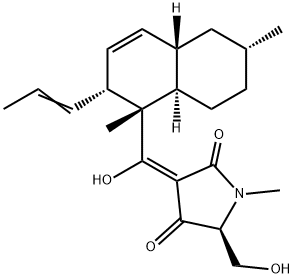
EQUISETIN
- CAS No.
- 57749-43-6
- Chemical Name:
- EQUISETIN
- Synonyms
- LS-64262;EQUISETIN;Citalopram Impurity 39;(3E,5S)-3-[[(1S,2R,4aS,6R,8aR)-1,6-dimethyl-2-[(E)-prop-1-enyl]-4a,5,6,7,8,8a-hexahydro-2H-naphthalen-1-yl]-hydroxymethylidene]-5-(hydroxymethyl)-1-methylpyrrolidine-2,4-dione;2,4-Pyrrolidinedione, 5-(hydroxymethyl)-3-[hydroxy[(1S,2R,4aS,6R,8aR)-1,2,4a,5,6,7,8,8a-octahydro-1,6-dimethyl-2-(1E)-1-propen-1-yl-1-naphthalenyl]methylene]-1-methyl-, (3E,5S)-
- CBNumber:
- CB1281192
- Molecular Formula:
- C22H31NO4
- Molecular Weight:
- 373.49
- MOL File:
- 57749-43-6.mol
- Modify Date:
- 2023/5/18 11:31:11
| Melting point | 117-118 °C |
|---|---|
| Boiling point | 556.0±50.0 °C(Predicted) |
| Density | 1.180±0.06 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Acetone (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.50±1.00(Predicted) |
| color | Pale Beige to Light Brown |
| Stability | Hygroscopic |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P312-P260-P280 |
| HS Code | 29339900 |
| Toxicity | mouse,LD50,intraperitoneal,63mg/kg (63mg/kg),Antimicrobial Agents and Chemotherapy. Vol. 5, Pg. 634, 1974. |
EQUISETIN Chemical Properties,Uses,Production
Description
Equisetin is a fungal metabolite that has been isolated from Fusarium. It inhibits HIV-1 integrase 3’ end-processing and strand transfer activities. Equisetin inhibits the ATPase activity of rat liver mitochondria and mitoplasts stimulated by 2,4-dinitrophenol (Dnp) in a concentration-dependent manner (IC50 = ~8 nM per mg of protein for both). It also inhibits ADP-stimulated respiration and the mitochondrial transport of ATP, inorganic phosphate, and succinate. Epiequisetin is phytotoxic and inhibits the germination of various seeds and growth of young seedlings.
Uses
The tetramic acid, equisetin, is produced by a number of species of Fusarium. Interest in equisetin emerged with reports of its inhibitory activity against HIV-1 integrase in vitro that was mechanistically distinct from previously described inhibitors. Equisetin inhibits 3' end-processing and strand transfer, as well as disintegration catalysed by either the full-length enzyme or the truncated integrase core.
EQUISETIN Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Shanghai Aladdin Bio-Chem Technology Co.,LTD | 400-400-6206333 18521732826 | China | 25013 | 65 | Inquiry |
| RD International Technology Co., Limited | 18024082417 | China | 9282 | 58 | Inquiry |
| Jiangsu Aikon Biopharmaceutical R&D co.,Ltd. | 025-66113011 18112977050 | China | 10529 | 58 | Inquiry |
| DC Chemicals | 021-58447131 13564518121 | China | 9412 | 58 | Inquiry |
| Guangzhou Yaoguang Technology Co., Ltd. | 13020159086 | China | 490 | 58 | Inquiry |





