PHENYL 2-(TRIMETHYLSILYL)ETHYL SULFONE

- CAS No.
- 73476-18-3
- Chemical Name:
- PHENYL 2-(TRIMETHYLSILYL)ETHYL SULFONE
- Synonyms
- (2-Phenylsulfonylethyl)trimethylsilane;PHENYL 2-(TRIMETHYLSILYL)ETHYL SULFONE;Trimethyl(2-(Phenylsulfonyl)Ethyl)Silane;1-(phenylsulfonyl)-2-(trimethylsilyl)ethane;PHENYL 2-(TRIMETHYLSILYL)ETHYL SULFONE, 99+%;Benzene, [[2-(trimethylsilyl)ethyl]sulfonyl]-;1-(Phenylsulfonyl)-2-(trimethylsilyl)ethane, 2-(Phenylsulfonyl)ethyltrimethylsilane, 2-(Trimethylsilyl)ethyl phenyl sulfone
- CBNumber:
- CB1284168
- Molecular Formula:
- C11H18O2SSi
- Molecular Weight:
- 242.41
- MOL File:
- 73476-18-3.mol
- Modify Date:
- 2023/7/10 8:28:02
| Melting point | 51-52 °C(lit.) |
|---|---|
| Boiling point | 347.4±34.0 °C(Predicted) |
| Density | 1.040±0.06 g/cm3(Predicted) |
| Flash point | >230 °F |
| solubility | sol all common ethereal, halocarbon, and hydrocarbon solvents. |
| form | solid |
| CAS DataBase Reference | 73476-18-3 |
PHENYL 2-(TRIMETHYLSILYL)ETHYL SULFONE Chemical Properties,Uses,Production
Chemical Properties
white fine crystalline powder
Physical properties
mp 52 °C.
Uses
(2-Phenylsulfonylethyl)trimethylsilane is widely used as reagent for the synthesis of mono- and 1,1-disubstituted alkenes via sulfone metalation, alkylation, and fluoride-induced elimination.
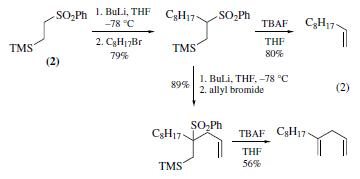
A sequence involving metalation, alkylation, and fluoride-induced elimination of benzenesulfinate allows the conversion of (2) to a terminal alkene. An analogous sequence involving a double alkylation of (2) provides a 1,1-disubstituted alkene (eq 2). The lithio derivative of (2) has also been used to prepare cyclopropylidene derivatives, homoallylic alcohols, and allyl silanes via the Julia alkenation.
Preparation
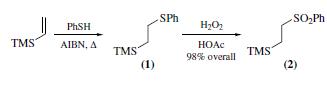
(2-phenylsulfonylethyl)trimethylsilane (2) is prepared by radical addition of thiophenol to vinyltrimethylsilane to give (2-phenylthioethyl)trimethylsilane (1), which is then oxidized with hydrogen peroxide).
Purification Methods
Dissolve it in Et2O, wash it with saturated HCO 3 followed by saturated NaCl, H2O and dried (MgSO4). Evaporation leaves residual crystals with m 52o. [Hsiao & Shechter Tetrahedron Lett 23 1963 1982, Bortolini et al. J Org Chem 53 2688 1985.]
PHENYL 2-(TRIMETHYLSILYL)ETHYL SULFONE Preparation Products And Raw materials
Raw materials
Preparation Products
PHENYL 2-(TRIMETHYLSILYL)ETHYL SULFONE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Chemistry | United States | 24072 | 58 | Inquiry | |
| Kanto Chemical Co., Inc. | +81 3 3663 7631 | Japan | 6762 | 74 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |
| 3B Scientific Corporation | 847.281.9822 | United States | 6744 | 47 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| CARBONE SCIENTIFIC CO.,LTD | +44(0)870 486 8629 | United Kingdom | 6682 | 30 | Inquiry |
| SIGMA-RBI | 800 736 3690 (Orders) | Switzerland | 6913 | 91 | Inquiry |
| Acros Organics | +32 14/57.52.11 | Belgium | 6780 | 81 | Inquiry |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | China | 96815 | 76 | Inquiry |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169085 | China | 40240 | 62 | Inquiry |
| Supplier | Advantage |
|---|---|
| Alfa Chemistry | 58 |
| Kanto Chemical Co., Inc. | 74 |
| Service Chemical Inc. | 71 |
| 3B Scientific Corporation | 47 |
| HONEST JOY HOLDINGS LIMITED | 54 |
| CARBONE SCIENTIFIC CO.,LTD | 30 |
| SIGMA-RBI | 91 |
| Acros Organics | 81 |
| J & K SCIENTIFIC LTD. | 76 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 62 |





