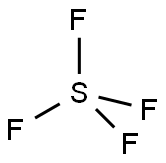
Sulfur tetrafluoride
- CAS No.
- 7783-60-0
- Chemical Name:
- Sulfur tetrafluoride
- Synonyms
- SF4;SULPHUR TETRAFLUORIDE;sulfur fluoride;Sulfur(iv) fluoride;Chebi:30495;Sulfur(4+) fluoride;Tetrafluoridosulfur;SULFUR TETRAFLUORIDE;Tetrafluorosulfurane;Schwefeltetrafluorid
- CBNumber:
- CB1286834
- Molecular Formula:
- F4S
- Molecular Weight:
- 108.06
- MOL File:
- 7783-60-0.mol
- Modify Date:
- 2024/12/18 14:07:02
| Melting point | −121.5-−120.5 °C(lit.) |
|---|---|
| Boiling point | −40.4 °C(lit.) |
| Density | 1.941 |
| vapor pressure | 140 psi ( 21 °C) |
| solubility | reacts with H2O |
| form | colorless gas |
| color | colorless |
| Water Solubility | decomposes in H2O [HAW93] |
| Merck | 13,9068 |
| CAS DataBase Reference | 7783-60-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfur tetrafluoride(7783-60-0) |
| EPA Substance Registry System | Sulfur tetrafluoride (7783-60-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS04,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H280-H330-H314 | |||||||||
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P410+P403-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501 | |||||||||
| Hazard Codes | T+,C,T | |||||||||
| Risk Statements | 14-26-34-37 | |||||||||
| Safety Statements | 26-36/37/39-38-45 | |||||||||
| RIDADR | UN 2418 2.3 | |||||||||
| OEL | Ceiling: 0.1 ppm (0.4 mg/m3) | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WT4800000 | |||||||||
| Hazard Note | Highly Toxic/Corrosive | |||||||||
| HazardClass | 2.3 | |||||||||
| Hazardous Substances Data | 7783-60-0(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Sulfur tetrafluoride Chemical Properties,Uses,Production
Chemical Properties
Sulfur tetrafluoride is a colorless gas with an odor like sulfur dioxide. Shipped as a liquefied compressed gas.
Uses
Compounds of interest in this group include sulfur hexafluoride (SF6) used as an electrical insulating material in circuit breakers, cables, capacitors, and transformers, and its degradation products, which are produced when electrical arcing occurs. The specific compounds produced depend on the arcing conditions. In anaerobic and anhydrous circumstances, sulfur tetrafluoride (SF4) is produced; if moisture is present, the tetrafluoride may hydrolyze to form thionyl fluoride (SOF2) and HF. Sulfuryl fluoride (SO2F2), disulfur decafluoride (S2F10), sulfur pentafluoride (SF5), and sulfur dioxide are also formed from sulfur hexafluoride. Some of these compounds are also produced as contaminants in the commercial production of sulfur hexafluoride by burning sulfur in fluorine gas, for example, sulfur tetrafluoride and disulfur decafluoride, as well as sulfur monofluoride (S2F2).
General Description
Sulfur tetrafluoride is a colorless gas with a distinct sulfur odor. Sulfur tetrafluoride is highly toxic by inhalation and a strong irritant to skin, eyes and mucous membranes. Sulfur tetrafluoride reacts vigorously with water and acids to yield toxic fluoride and sulfur oxide fumes and an acidic solution. Sulfur tetrafluoride is heavier than air. Under prolonged exposure to fire or intense heat the containers may violently rupture or rocket. Sulfur tetrafluoride is used as a fluoridizing agent and as an oil repellent.
Air & Water Reactions
Violent reaction with water. Sulfur tetrafluoride reacts vigorously with water and acids to yield toxic fluoride and sulfur oxide fumes and an acidic solution.
Reactivity Profile
Sulfur tetrafluoride is a highly toxic and corrosive gas. On contact with water, steam, or mineral acids Sulfur tetrafluoride decomposes and produces toxic and highly irritating fumes. When heated to decomposition Sulfur tetrafluoride emits very toxic fluoride and sulfur oxides fumes [Lewis, 3rd ed., 1993, p. 1197]. Explosively violent reactions with 2-(hydroxymethyl)furan or 2-methyl-3-butyn-2-ol even below -50° C have been recorded [Bretherick, 5th ed., 1995, p. 1432]. Ignition or explosion may occur on contact with dioxygen difluoride even below -100° C [Streng, A. G., Chem. Rev., 1963, 63, p. 615].
Hazard
High by inhalation, strong irritant to eyes, mucous membranes, and upper respiratory tract irritant. Lung damage.
Health Hazard
Sulfur tetrafluoride is highly toxic by inhalation; it is a strong irritant to eyes and mucous membranes. Poisonous; may be fatal if inhaled. Contact may cause burns to skin and eyes. Contact with liquid may cause frostbite.
Fire Hazard
Container may explode in heat of fire. When heated to decomposition, Sulfur tetrafluoride emits very toxic fumes of fluorides and sulfur oxides. Reacts violently with water. Sulfur tetrafluoride is decomposed by concentrated sulfuric acid. Thermostable to 1112F.
Safety Profile
Poison by inhalation. A powerful irritant. Will react with water, steam, or acids to yield toxic and corrosive fumes. Incompatible with dioxygen difluoride. When heated to decomposition it emits very toxic fumes of Fand SOx. See also FLUORIDES.
Potential Exposure
Sulfur tetrafluoride is used as a selective fluorinating agent in making water-repellent and oil repellent materials and lubricity improvers. It is also used as a pesticide intermediate.
Shipping
UN2418 Sulfur tetrafluoride, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. Forbidden to be transported by any aircraft or by rail tank car.
Incompatibilities
Keep away from moisture, concentrated sulfuric acid, dioxygen difluoride. Reacts vigorously with water, alcohols and acids releasing toxic fluoride, sulfur oxide fumes and forming a corrosive acid solution. Readily hydrolyzed by moisture, forming hydrofluoric acid, thionyl fluoride. Attacks glass, ceramic, concrete
Waste Disposal
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillabletype cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.
Sulfur tetrafluoride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21628 | 55 | Inquiry |
| Fluoropharm Co., Ltd. | +86-0571-85586753 +86-13336034509 | China | 1445 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29859 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | China | 23541 | 58 | Inquiry |
| CR Corporation Limited | +8613062833949 | China | 8647 | 58 | Inquiry |
| CD Chemical Group Limited | +8615986615575 | China | 20342 | 58 | Inquiry |
| changzhou huayang technology co., ltd | +8615250961469 | China | 9718 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8772 | 58 | Inquiry |
Related articles
- Is SF4 Polar or Nonpolar?
- ?Sulfur tetrafluoride (SF4) is a polar molecule. It is made up of four fluorine (F) atoms bonded to a sulfur (S) atom. Due to ....
- Dec 22,2023
7783-60-0(Sulfur tetrafluoride )Related Search:
1of4
chevron_right




