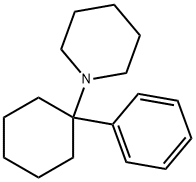
PHENCYCLIDINE
- CAS No.
- 77-10-1
- Chemical Name:
- PHENCYCLIDINE
- Synonyms
- PCP;C07575;HUMPCP;Phencylidine;PHENCYCLIDINE;PrCP active human;Phencyclidine (pcp);Pcp (phencyclidine);prolylcarboxypeptidase;Phencyclidine solution
- CBNumber:
- CB1310072
- Molecular Formula:
- C17H25N
- Molecular Weight:
- 243.39
- MOL File:
- 77-10-1.mol
- Modify Date:
- 2023/4/23 13:52:06
| Melting point | 46.5℃ |
|---|---|
| Boiling point | bp1.0 135-137° |
| Density | 0.9762 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| Flash point | 11 °C |
| storage temp. | −20°C |
| pka | pKa 8.5 (Uncertain) |
| color | Colorless crystals |
| EPA Substance Registry System | Piperidine, 1-(1-phenylcyclohexyl)- (77-10-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225-H301+H311+H331-H370 |
| Precautionary statements | P210-P260-P280-P301+P310-P311 |
| Hazard Codes | F,T |
| Risk Statements | 23/24/25-39/23/24/25-11-61 |
| Safety Statements | 36/37-45-16-53 |
| RIDADR | 2811 |
| WGK Germany | 1 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Toxicity | LD50 oral in mouse: 75mg/kg |
PHENCYCLIDINE Chemical Properties,Uses,Production
Uses
Anesthetic.
Definition
ChEBI: A member of the class of piperidines that is piperidine in which the nitrogen is substituted with a 1-phenylcyclohexyl group. Formerly used as an anaesthetic agent, it exhibits both hallucinogenic and neurotoxic effects.
General Description
Phencyclidine was introduced as a dissociative anestheticfor animals. Its close structural relative ketamine is still soused and may be used in humans. In humans,PCP produces a sense of intoxication, hallucinogenic experiencesnot unlike those produced by the anticholinergic hallucinogens,and often, amnesia.
The drug affects many systems, including those of NE,DA, and 5-HT. It has been proposed that PCP (and certainother psychotomimetics) produces a unique pattern of activationof ventral tegumental area dopaminergic neurons.Itblocks glutaminergic N-methyl-D-aspartate receptors.Thisaction is the basis for many of its CNS effects. PCP itself appearsto be the active agent. The psychotic state produced bythis drug is also cited as a better model than amphetaminepsychosis for the psychotic state of schizophrenia.
Pharmacology
PCP acts as a biocide through its ability to uncouple mitochondrial oxidative phosphorylation.
Safety Profile
Poison by intraperitoneal route. Experimental reproductive effects. Caution: This is a controlled substance (depressant) listed in the U.S. Code of Federal Regulations, Title 21 Part 1308.12 (1985). The ethylamine, pyrrolidine and thiophene analogs are l
Metabolic pathway
When mice and rats are administered phencyclidine intraperitoneally, several hydroxylated metabolites are identified in the urine. A new metabolite, 1-phenyl-1- (1-piperidinyl-3-ol)cyclohexane, is identified in the urine and liver microsomal preparations.
Metabolism
Pentachlorophenolwas metabolized in rats
by conjugation with glucuronic acid and eliminated as
the glucuronide. P450 catalyzed oxidative dechlorination
also occurred to form tetrachlorohydroquinone, and this
was conjugated to form a monoglucuronide representing
27% of the dose administered. Other metabolites
have been reported, including isomeric tetrachlorophenols,
tetrachlorocatechol and tetrachlororesorcinol. Trace
amounts of benzoquinones were also noted.
Metabolites in female rats were tetrachloromonophenols,
diphenols, and hydroquinones.
PHENCYCLIDINE Preparation Products And Raw materials
Raw materials
Preparation Products
77-10-1(PHENCYCLIDINE)Related Search:
1of4
chevron_right




