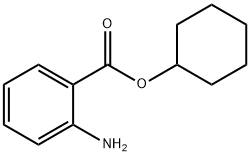
CYCLOHEXYL ANTHRANILATE
- CAS No.
- 7779-16-0
- Chemical Name:
- CYCLOHEXYL ANTHRANILATE
- Synonyms
- FEMA 2350;CYCLOHEXYL ANTHRANILATE;Cyclohexyl 2-aminobenzoate;2-Aminobenzoicacidcyclohexylester;2-amino-benzoicacicyclohexylester;Anthranilic acid, cyclohexyl ester;Benzoic acid, 2-amino-, cyclohexyl ester
- CBNumber:
- CB1781384
- Molecular Formula:
- C13H17NO2
- Molecular Weight:
- 219.28
- MOL File:
- 7779-16-0.mol
- Modify Date:
- 2024/12/18 14:07:02
| Boiling point | 134 °C |
|---|---|
| Density | 1,11 g/cm3 |
| FEMA | 2350 | CYCLOHEXYL ANTHRANILATE |
| pka | 2.17±0.10(Predicted) |
| Specific Gravity | 1.11 |
| Odor | at 100.00 %. mild neroli orange blossom |
| Odor Type | fruity |
| JECFA Number | 1541 |
| LogP | 4.10 |
| CAS DataBase Reference | 7779-16-0(CAS DataBase Reference) |
| EPA Substance Registry System | Benzoic acid, 2-amino-, cyclohexyl ester (7779-16-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H335-H315 |
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P |
| Hazard Codes | Xi |
| HazardClass | IRRITANT |
CYCLOHEXYL ANTHRANILATE Chemical Properties,Uses,Production
Chemical Properties
Cyclohexyl anthranilate has a faint, fruity, orange blossom-like odor, and a sweet, fruity, grape-like taste.
Uses
Cyclohexyl anthranilate is used in flavor compositions for imitation Grape, Apple, Banana, etc. Concentration in the finished product is normally mere traces.
Preparation
From isatoic anhydride and cyclohexanol.
General Description
Viscous yellow liquid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
An amine and ester. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Fire Hazard
CYCLOHEXYL ANTHRANILATE is probably combustible.
CYCLOHEXYL ANTHRANILATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49732 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30231 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8772 | 58 | Inquiry |
| DAYANG CHEM (HANGZHOU) CO.,LTD | +8617705817739 | China | 53881 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131957 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6831 | 75 | Inquiry |
| Penta Manufacturing Company | 973 740 2300 | United States | 5076 | 65 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 029-029-81148696 18161915376 | China | 9799 | 58 | Inquiry |
| Yantai Shengkailun Chemical Technology Co., Ltd. | 13356901049 | China | 9309 | 58 | Inquiry |
| Absin Bioscience Inc. | 021-38015121-8802 | CHINA | 6208 | 58 | Inquiry |
7779-16-0(CYCLOHEXYL ANTHRANILATE)Related Search:
1of4
chevron_right




