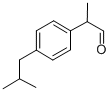
2-(4-isobutylphenyl)propionaldehyde
- CAS No.
- 51407-46-6
- Chemical Name:
- 2-(4-isobutylphenyl)propionaldehyde
- Synonyms
- Ibuprofen Aldehyde;Ibuprofen Impurity 75;2-(4-Isobutylphenyl)propanal;2-(4-isobutylphenyl)propionaldehyde;p-(2-Methylpropyl)hydratropaldehyde;2-[4-(2-Methylpropyl)phenyl]propanal;α-Methyl-4-isobutylbenzeneacetaldehyde;(2RS)-2-[4-(2-Methylpropyl)phenyl]propan-1-al;A-METHYL-4-(2-METHYLPROPYL)BENZENEACETALDEHYDE;α-Methyl-4-(2-methylpropyl)benzeneacetaldehyde
- CBNumber:
- CB1902754
- Molecular Formula:
- C13H18O
- Molecular Weight:
- 190.28
- MOL File:
- 51407-46-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/21 13:33:47
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H319-H315 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362-P264-P270-P301+P312-P330-P501 |
2-(4-isobutylphenyl)propionaldehyde Chemical Properties,Uses,Production
Uses
2-(4-Isobutylphenyl)propanal is an impurity of the drug Ibuprofen (I140000), a selective cyclooxygenase inhibitor that also inhibits PGH synthase-1 and PGH synthase-2 with comparable potency.
Production Methods
Numerous methods are known for the synthesis of 2-(4-isobutylphenyl)propionaldehyde. It can be prepared by reaction of p-isobutylacetophenone with methyl chloroacetate using sodium methoxide as catalyst, followed by reaction of the glycidic ester with BF3 to give 2- hydroxy-3-(4-isobutylphenyl)-3-butenoic acid methyl ester, which is subsequently treated with mineral acid Avariant of the process is the hydrolysis of the intermediately formed glycidic ester with alkali to give the corresponding salt of glycidic acid, which is then decarboxylated. In addition, 2-(4-isobutylphenyl)propanal is formed by isomerization of 2-(4-isobutylphenyl)- 2-methyloxirane on Al2O3-SiO2 or anhydrous ZnCl2, by reaction of 1-(4-isobutylphenyl)- 1-chloroethane with dimethylformamide in the presence of Li or Na in tetrahydrofuran, and in good yields by Rh- or Co-catalyzed hydroformylation of p-isobutylstyrene.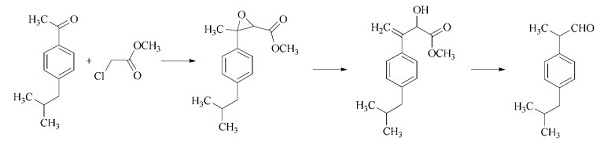
2-(4-isobutylphenyl)propionaldehyde Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Chemicea Pharmaceuticals Pvt Ltd | +91-9881765045/+91-8796656135 | Pune, India | 825 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | China | 3978 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | China | 11922 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43340 | 58 | Inquiry |
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366; 13670046396 | China | 24342 | 58 | Inquiry |
51407-46-6(2-(4-isobutylphenyl)propionaldehyde)Related Search:
1of4
chevron_right




