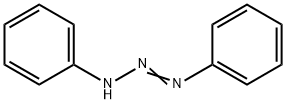
Diazoaminobenzene
- CAS No.
- 136-35-6
- Chemical Name:
- Diazoaminobenzene
- Synonyms
- DAAB;Cellofor;cellofor(czech);Diazoaminobenzen;Diazoaminobenzol;DIAZOAMINOBENZENE;anilinoazobenzene;benzeneazoaniline;Benzene azoanilide;benzenediazoanilide
- CBNumber:
- CB2344713
- Molecular Formula:
- C12H11N3
- Molecular Weight:
- 197.24
- MOL File:
- 136-35-6.mol
- Modify Date:
- 2024/12/18 14:08:52
| Melting point | 96°C |
|---|---|
| Boiling point | 324.34°C (rough estimate) |
| Density | 1.1793 (rough estimate) |
| vapor pressure | >1 Pa |
| refractive index | 1.6500 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | 0.5g/l insoluble |
| pka | 1.00±0.30(Predicted) |
| form | powder |
| color | Orange |
| Water Solubility | 499.8mg/L(room temperature) |
| InChIKey | ALIFPGGMJDWMJH-UHFFFAOYSA-N |
| CAS DataBase Reference | 136-35-6(CAS DataBase Reference) |
| EPA Substance Registry System | 1-3-Diphenyltriazine (136-35-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H335-H302+H312+H332-H315 |
| Precautionary statements | P261-P271-P280 |
| Hazard Codes | E,Xn |
| Risk Statements | 1-5-20/21/22-36/37/38 |
| Safety Statements | 15-26-27-36/37/39 |
| WGK Germany | WGK 3 highly water endangering |
| RTECS | XY2625000 |
| HazardClass | IRRITANT |
| HS Code | 29270000 |
| Hazardous Substances Data | 136-35-6(Hazardous Substances Data) |
Diazoaminobenzene Chemical Properties,Uses,Production
Description
Diazoaminobenzene (DAAB) is an aromatic amine that is a suspected carcinogen. It is harmful if inhaled, ingested, or absorbed through the skin. It causes skin irritation and severe irritation to eyes. DAAB can be made by diazotizing aniline dissolved in hydrochloric acid with sodium nitrite and then adding a concentrated solution of sodium acetate. DAAB is listed in the US Environmental Protection Agency’s Toxic Substances Control Act Inventory. DAAB has three major use areas: intermediate, complexing agent, and polymer additive. Use as an intermediate is reported in several industry sectors, including organic synthesis, dye manufacture, and agrochemical manufacture (insecticides). DAAB is also a versatile metal complexing agent. A series of metabolism studies in rodents and human liver slices, electron spin resonance spectroscopy studies, short-term dermal toxicity studies in rodents, and acute bone marrow micronucleus studies in mice demonstrated that DAAB is metabolized and shares similar genotoxic and toxicological properties to the known human carcinogen, benzene, and the known rodent carcinogen, aniline.
Chemical Properties
ochre powder
Uses
Diazoaminobenzene is used as a chemical intermediate, complexing agent, and polymer additive (Mathews and De Costa 1999). It has uses associated with organic synthesis and dye and insecticide manufacture (Lewis 1997), and it is an effective dopant for laser ablation (micro-machining) of polymethylmethacrylate (Bolle et al. 1990). Diazoaminobenzene has been identified as a low-level contaminant in the dyes D&C red no. 33, FD&C yellow no. 5 (tartrazine), and FD&C yellow no. 6; all three are permitted for use in drugs and cosmetics, and the latter two are permitted in food (FDA 2010).
General Description
Orange solid.
Air & Water Reactions
Dust can be explosive when suspended in air at specific concentrations. Insoluble in water.
Reactivity Profile
1,3-DIPHENYLTRIAZENE is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. 1,3-DIPHENYLTRIAZENE explodes when heated to above 150°C. A mixture of the triazine and acetic anhydride exploded violently upon warming, Ber., 1891, 24, 4160.
Health Hazard
ACUTE/CHRONIC HAZARDS: 1,3-DIPHENYLTRIAZENE may explode if subjected to severe shock or heat.
Fire Hazard
Flash point data for 1,3-DIPHENYLTRIAZENE are not available, however, 1,3-DIPHENYLTRIAZENE is probably combustible.
Safety Profile
Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Strongly explosive when shocked or heated to 98'C. Mixture with acetic anhydride explodes when warmed. When heated to decomposition it emits toxic fumes of NOx,.
Carcinogenicity
Diazoaminobenzene is reasonably anticipated to be a human carcinogen based on (1) evidence from studies in experimental animals andwith human tissue demonstrating that diazoaminobenzene is metabolized to benzene, a known human carcinogen, and (2) evidence that diazoaminobenzene causes genetic damage. Studies in rats and mice have shown that the metabolism of diazoaminobenzene to benzene is quantitative. Benzene was listed in the First Annual Report onCarcinogens in 1980 based on human epidemiological studies dem-causes cancer at numerous tissue sites in rodents.
Environmental Fate
DAAB is a respiratory tract, skin, and eye irritant. DAAB yields benzene and aniline as metabolites. The proposed metabolic pathway forDAAB is that it is cleaved reductively by liver enzymes or gut flora to form aniline, benzene, and nitrogen. DAAB metabolism also results in the formation of a reactive phenyl radical, which could account for an additional risk of toxicity or carcinogenicity. The erythrocyte and lymphoid systems are major targets of DAAB toxicity. Induction of lymphoid atrophy of the thymus and other lymphoid tissues were observed, as well as methemoglobin formation, accompanying anemia, increased spleen weights, and regenerative hematopoiesis. Analysis of organ weights indicated possible chemical-related effects in the thymus, heart, spleen, kidney, and liver of rats and/or mice. Increases in the incidences of several skin lesions, including hyperplasia of the epidermis and hair follicles, and inflammation in rats and mice and ulceration in female mice were observed.
Diazoaminobenzene Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29808 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8772 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | China | 18137 | 58 | Inquiry |
| Aladdin Scientific | United States | 57505 | 58 | Inquiry | |
| DAYANG CHEM (HANGZHOU) CO.,LTD | +8617705817739 | China | 53881 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6831 | 75 | Inquiry |
| Jiangsu Raien Environmental Protection Technology Co., Ltd | 0513-0513-66814573 16651336298 | China | 4349 | 58 | Inquiry |
| Biosynth Biological Technology (Suzhou) Co Ltd | 51288865780 | China | 233051 | 58 | Inquiry |
| Jiangsu Runfeng Synthetic Technology Co., Ltd. | 18551497631 18551497631 | China | 5058 | 58 | Inquiry |
Related articles
- Uses of Diazoaminobenzene
- Diazoaminobenzene (DAAB) is an aromatic amine that is a suspected carcinogen. It is harmful if inhaled, ingested, or absorbed ....
- Jan 17,2022
136-35-6(Diazoaminobenzene)Related Search:
1of4
chevron_right




