1-BUTEN-3-YNE
- CAS No.
- 689-97-4
- Chemical Name:
- 1-BUTEN-3-YNE
- Synonyms
- Butenyne;1-Butenyne;Buten-3-yne;1-BUTEN-3-YNE;1-Butyn-3-ene;ethynyl-ethen;Ethynylethene;3-BUTEN-1-YNE;VINYLACETYLENE;1-Butene-3-yne
- CBNumber:
- CB2454902
- Molecular Formula:
- C4H4
- Molecular Weight:
- 52.07
- MOL File:
- 689-97-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/21 16:32:15
| Melting point | 203 °C(Solv: water (7732-18-5)) |
|---|---|
| Boiling point | 3°C |
| Density | 0.6800 |
| refractive index | 1.4161 |
| EPA Substance Registry System | Vinyl acetylene (689-97-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS04 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H280 | |||||||||
| Precautionary statements | P410+P403-P210-P377-P381-P403 | |||||||||
| RIDADR | 1954 | |||||||||
| HazardClass | 2.1 | |||||||||
| NFPA 704 |
|
1-BUTEN-3-YNE Chemical Properties,Uses,Production
Description
Vinylacetylene, also known as butenyne, monovinylacetylene, 1-butene-3-yne, 1-butyn- 3-ene, 3-buten-1-yne, buten-3-yne, ethynylethene, 1-butenyne and vinylethyne, is a derivative of acetylene.
Chemical Properties
Vinylacetylene is a gas with a smell similar to acetylene. The melting point is –118
to –120°C, the boiling point is 5.5 °C under atmospheric pressure, and the boiling
point of vinylacetylene under different pressures is shown in Table 7.1.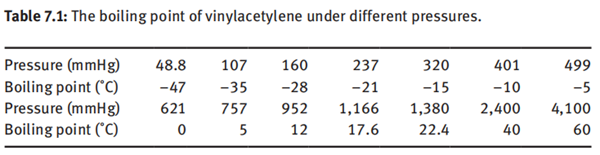
Uses
Vinylacetylene is mainly used for the production of chloroprene, leaf alcohol and methanol adhesives. It is also used for the production of divinyl ether and methyl vinyl (methyl) ketone-based polymer.
General Description
A colorless gas or liquid. Derived by the dimerization of acetylene. Used in the synthesis of neoprene and for other organic syntheses.
Air & Water Reactions
Highly flammable. Oxidizes in air to form unstable peroxides that may explode spontaneously.
Reactivity Profile
1-BUTEN-3-YNE may react vigorously with strong oxidizing agents. May react exothermically with reducing agents In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions. Forms an explosive salt with silver nitrate. Thermally unstable, under pressure, in combination with 1,3-butadiene [Loss Prev., 1971, 5, 67].
Synthesis
Acetylene is dimerized in the presence of hydrochloric acid solution of cuprous chloride and ammonium chloride to obtain vinylacetylene.
1-BUTEN-3-YNE Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Vadilal Chemicals Limited | +91-7948936937 +91-7203030735 | Gujarat, India | 39 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 7726 | 58 | Inquiry |
| DWS Specialty Gas Co., Ltd | 131-9467-7939 4001882517 | China | 454 | 58 | Inquiry |
| Foshan Zhicheng Gas Co., Ltd., | 18098161577 | China | 168 | 58 | Inquiry |
| Maotu Gas Equipment (Shanghai) Co. LTD | 17301833415 | China | 271 | 58 | Inquiry |
| Hubei Xinkang Pharmaceutical Chemical Co., Ltd | 027-59308705 18871579363 | China | 9954 | 58 | Inquiry |
| Shanghai Xx-lab New Materials Co., Ltd | 13585834793 | China | 1428 | 58 | Inquiry |
| Wuhan Linglingjiu Biotechnology Co., Ltd | 15623309010 | China | 1657 | 58 | Inquiry |
| Baoji Didu Pharmaceutical and Chemical Co., Ltd | 029-81148929 13186179623 | China | 10011 | 58 | Inquiry |
| Shanghai Jasper Gas Co., Ltd. | 021-13218776790 13218776790 | China | 443 | 58 | Inquiry |
Related articles
- Process for manufacture of vinylacetylene
- Acetylene is dimerized in the presence of hydrochloric acid solution of cuprous chloride and ammonium chloride to obtain vinyl....
- Mar 21,2024
689-97-4(1-BUTEN-3-YNE)Related Search:
1of4
chevron_right




