OXAMNIQUINE(200MG)DISCONTINUED
- CAS No.
- 21738-42-1
- Chemical Name:
- OXAMNIQUINE(200MG)DISCONTINUED
- Synonyms
- Vansil;mansil;uk4261;uk4271;NSC 352888;oxamniquine;UK 4271/Vansil;oxamniquinum(inn-latin);oxamniquina(inn-spanish);OXAMNIQUINE(200MG)DISCONTINUED
- CBNumber:
- CB2505817
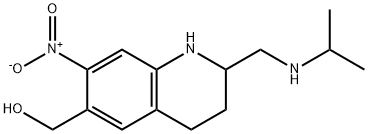
- Molecular Formula:
- C14H21N3O3
- Molecular Weight:
- 279.33484
- MOL File:
- 21738-42-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/10/23 13:36:13
| Melting point | 147-149° |
|---|---|
| Boiling point | 422.1°C (rough estimate) |
| Density | 1.1119 (rough estimate) |
| refractive index | 1.5700 (estimate) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 3.28 ± 0.07;9.53(H2O,t =25) (Uncertain) |
| color | Light-orange powder |
| BCS Class | 4/2 |
| Stability | Hygroscopic |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H413 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| Hazardous Substances Data | 21738-42-1(Hazardous Substances Data) |
| Toxicity | LD50 in mice, rabbits (mg/kg): >2000, >1000 i.m., 1300, 800 orally (Foster) |
OXAMNIQUINE(200MG)DISCONTINUED Chemical Properties,Uses,Production
Description
Oxamniquine was originally investigated in the 1960s and was found to have limited antiprotozoal activity, with activity against Schi stosoma mansoni but no activity against the other two schistosomal organisms. In addition, the drug is stage specific, with activity against cercariae and very young schistosomula and adult worms. For reasons that remain unknown, the drug is more effective against adult male worms than against female worms. The drug has structural similarity to hycanthone, which is no longer used because of severe toxicity and teratogenic effects.
Uses
Antischistosomal.
Indications
Oxamniquine (Vansil) is a tetrahydroquinoline that
stimulates parasite muscular activity at low concentrations
but causes paralysis at higher concentrations. The
drug may act by esterification and binding of DNA,
leading to the death of the schistosome by interruption
of its nucleic acid and protein synthesis. The fluke may
esterify oxamniquine to produce a reactive metabolite
that alkylates parasite DNA. Resistance results from
absent or defective esterifying activity of the drug.
Oxamniquine has a restricted range of efficacy, being
active only against S. mansoni infections.
Oxamniquine is given orally and is readily absorbed
from the intestinal tract. Peak concentrations in plasma
are obtained in about 3 hours. The drug is excreted in
urine mostly as a 6-carboxyl derivative.
Side effects include CNS toxicity with unsteadiness
and occasionally seizures, especially in patients with a
history of seizures. It is contraindicated in pregnancy.
Definition
ChEBI: A member of the class of quinolines that is 1,2,3,4-tetrahydroquinoline which is substituted at positions 2, 6, and 7 by (isopropylamino)methyl, hydroxymethyl, and nitro groups, respectively.
Antimicrobial activity
Activity is restricted to Schistosoma mansoni. Some strains, particularly those in Egypt and Southern Africa, require higher doses for efficacy owing to innate tolerance.
Pharmaceutical Applications
A synthetic quinolinemethanol, available for oral administration.
Mechanism of action
Oxamniquine is activated via esterification to a biological ester that spontaneously dissociates to an electrophile, which alkylates the helminth DNA, leading to irreversible inhibition of nucleic acid metabolism. Resistant helminths do not esterify oxamniquine; therefore, activation does not occur. Other metabolic reactions consist of oxidative reactions, leading to inactivation. The metabolites are excreted primarily in the urine.
Pharmacokinetics
It is rapidly absorbed after oral administration, achieving a peak concentration of 0.3–2.5 mg/L 1–3 h after an oral dose of 15 mg/kg body weight. Peak levels following intramuscular treatment at 7.5 mg/kg generally do not exceed 0.15 mg/L. It is extensively metabolized to biologically inactive 6-carboxylic and 2-carboxylic acid derivatives, which are excreted in the urine, mostly within 12 h.
Clinical Use
Infection with S. mansoni
Side effects
Dizziness, sleepiness, nausea and headache occur frequently. Other side effects are probably due to the death and disintegration of the worms in the liver. Following treatment, urine may become red.
Safety Profile
Poison by ingestion, intraperitoneal and intramuscular routes. Human mutation data reported. An antischistosomal agent. When heated to decomposition it emits toxic fumes of NOx.
OXAMNIQUINE(200MG)DISCONTINUED Preparation Products And Raw materials
Raw materials
Preparation Products
OXAMNIQUINE(200MG)DISCONTINUED Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 22854 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49734 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; | China | 35425 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +8613564774135 | United States | 19881 | 58 | Inquiry |
| Merck KGaA | 21-20338288 | China | 292394 | 58 | Inquiry |
| BOC Sciences | USA | 0 | 65 | Inquiry | |
| Hubei Enxing Biotechnology Co., Ltd | 16621771607 16621771607 | China | 8176 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 029-029-81148696 18161915376 | China | 9799 | 58 | Inquiry |





