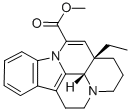
apovincamine
- CAS No.
- 4880-92-6
- Chemical Name:
- apovincamine
- Synonyms
- Apovincamina;apovincamine;Apovincaminum;Vinpocetine-2;Unii-504R182zx7;Einecs 225-491-0;cis-ApovincaMine;(+)-trans-Cavinton;(+)-trans-Cavinton;Apovincaminum [latin]
- CBNumber:
- CB2890058
- Molecular Formula:
- C21H24N2O2
- Molecular Weight:
- 336.43
- MOL File:
- 4880-92-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/8 18:06:34
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| HS Code | 2939800000 |
apovincamine price More Price(1)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR3634 | Vinpocetine Related Compound B certified reference material, pharmaceutical secondary standard | 4880-92-6 | 50MG | ₹71315.1 | 2022-06-14 | Buy |
| Product number | Packaging | Price | Buy |
|---|---|---|---|
| PHR3634 | 50MG | ₹71315.1 | Buy |
apovincamine Chemical Properties,Uses,Production
Originator
Apovincamine,GC Promochem
Manufacturing Process
Apovincamine is semisynthethetic derivative of (+)-vincamine. Vincamine is
alkaloid of Vinca minor. Alcoloid of Tabernaemontana rigida (Apocynaceae)
also is used as raw material for preparing of apovincamine. (+)- Vincamine
was transformed into oxime ester by reaction with NaNO2 in acetic acid at 0°C
-(+/-)-methyl-3-((12bR)-1-ethyl-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-
a]quinolizin-1-yl)-2-(hydroxyimino)propanoate, which was resolved on the
isomers with dibenzoyl D-tartratic acid. (-)-Methyl 3-((12bR)-1-ethyl-
1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-1-yl)-2-
(hydroxyimino)propanoate was re-crystallized from methanol to afford (-)-
methyloxime ester, MP: 195°C.
2 g of above oxime methyl ester was heated in mixture of methanol (37.5 ml)
and conc. H2SO4 (13.5 ml) on water bath for 1 hour. The solution was poured
into ice-water (80 ml), basified with conc. NH4OH to pH 9, and extracted with
CH2Cl2 (3x20 ml). The combined extracts were dried (MgSO4), filtered,
evaporated in vacuum and the residue was re-crystallized from MeOH (5 ml)
to yield 1.32 g (72.5%) of (+)-apovincamine, MP: 160°-162°C.
Therapeutic Function
Vasodilator
apovincamine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | 18192627656 | China | 3002 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | China | 11927 | 58 | Inquiry |
| PANNACEAN(HENAN)MEDICINE SCIENCE TECHNOLOGIES LTD | 0371-55159968 18137130222 | China | 527 | 58 | Inquiry |
| ROSEWA HOLDING GROUP CO.,LIMITED | +86-023-62871537 | China | 2844 | 58 | Inquiry |
| cjbscvictory | 13348960310 13348960310 | China | 10000 | 58 | Inquiry |
4880-92-6(apovincamine)Related Search:
1of4
chevron_right




