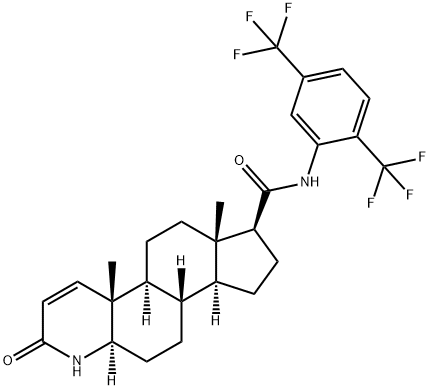
Dutasteride
- CAS No.
- 164656-23-9
- Chemical Name:
- Dutasteride
- Synonyms
- Avodart;Avolve;Duagen;(5α,17β)-;GI 198745;utasteride;DUTASTERIDE;Dutarantine;Dutasteride API;Dutasteride CRS
- CBNumber:
- CB3254628
- Molecular Formula:
- C27H30F6N2O2
- Molecular Weight:
- 528.53
- MOL File:
- 164656-23-9.mol
- Modify Date:
- 2024/9/18 10:41:17
| Melting point | 242-250°C |
|---|---|
| Boiling point | 620.3±55.0 °C(Predicted) |
| Density | 1.303±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble2mg/mL, clear |
| pka | 13.32±0.70(Predicted) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D +18 to +24°, c = 1 in chloroform-d |
| BCS Class | 2/4 |
| InChIKey | JWJOTENAMICLJG-QWBYCMEYSA-N |
| SMILES | N1[C@@]2([H])[C@@](C)([C@@]3([H])CC[C@@]4(C)[C@]([H])([C@]3([H])CC2)CC[C@@H]4C(NC2=CC(C(F)(F)F)=CC=C2C(F)(F)F)=O)C=CC1=O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H351-H360FD |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| HS Code | 2937290000 |
Dutasteride price More Price(2)
Dutasteride Chemical Properties,Uses,Production
Description
Dutasteride is a synthetic 4-azasteroid compound. It is a dual inhibitor of type 1 and 2 isoforms of 5α-reductase unlike finasteride, the first marketed 5α-reductase inhibitor, which only acts on type 2 isozyme. Dutasteride is a 3-fold greater inhibitor of type-2 5α-reductase than finasteride in men and has greater effect on the type-l than on type-2 isozyme. In animal models,dutasteride exhibited superior efficacy and pharmacokinetics compared to finasteride. In patients with benign prostate hyperplasia, administration of dutasteride was shown to dose-dependently decrease serum dihydrotestosterone levels with greater efficacy as compared to finasteride (95% vs 67%). Serum testosterone levels increased with both active drugs, in conjunction with dihydrotestosterone suppression but remained within normal ranges. In long term studies, in men with moderate to severe benign prostate hyperplasia, once daily dutasteride significantly reduced prostate volume, reduced the risk of acute urinary retention and surgery by 57% and improved lower urinary tract symptoms and urinary flow measurements.
Chemical Properties
Dutasteride is a white to pale yellow powder It is soluble in ethanol (44 mg/mL), methanol (64 mg/mL), and polyethylene glycol 400 (3 mg/mL), but it is insoluble in water.
Uses
Dutasteride is a dual inhibitor of 5a-reductase isoenzymes type 1 and 2; structurally related to Finasteride. Dutasteride is used in the treatment of benign prostatic hyperplasia. Dutasteride is associated with a low rate of transient serum aminotransferase elevations, but has yet to be linked to instances of clinically apparent acute liver injury.
Definition
ChEBI: Dutasteride is an aza-steroid that is inasteride in which the tert-butyl group is replaced by a 2,5-bis(trifluoromethyl)phenyl group. A synthetic 4-azasteroid, dutasteride is a selective inhibitor of both the type 1 and type 2 isoforms of steroid 5alpha-reductase, an intracellular enzyme that converts testosterone to 5alpha-dihydrotestosterone. Dutasteride is used for the treatment of symptomatic benign prostatic hyperplasia in men with an enlarged prostate gland. It has a role as an EC 1.3.1.22 [3-oxo-5alpha-steroid 4-dehydrogenase (NADP(+))] inhibitor and an antihyperplasia drug. It is an aza-steroid, a member of (trifluoromethyl)benzenes and a delta-lactam. It derives from a hydride of a 5alpha-androstane.
Biological Functions
Similar to finasteride, dutasteride is a competitive and mechanism-based inhibitor not only of type 2 but also of type 1 5α-reductase isoenzymes, with which stable enzyme-NADP adduct complexes are formed, inhibiting the conversion of testosterone to DHT. The suppression of both type 1 and type 2 isoforms results in greater and more consistent reduction of plasma DHT than that observed for finasteride. The more effective dual inhibition of type 1 and type 2 5α-reductase isoforms lowers circulating DHT to a greater extent than with finasteride and shows advantages in treating BPH and other disease states (e.g., prostate cancer) that are DHT-dependent.
Pharmacokinetics
The maximum effect of 0.5 mg daily doses of dutasteride on the suppression of DHT is dose-dependent and is observed within 1 to 2 weeks. After 2 weeks of 0.5 mg daily dosing, median plasma DHT concentrations were reduced by 90%, and after 1 year, the median decrease in plasma DHT was 94%. The median increase in plasma testosterone was 19% but remained within the physiological range. The drug also reduced serum prostatic specific antigen by approximately 50% at 6 months and total prostate volume by 25% at 2 years. Dutasteride produced improvements in quality of life and peak urinary flow rate and reduction of acute urinary retention without the need for surgery.
Clinical Use
Dutasteride belongs to azasteriod class of compounds and function as a 5α-reductase inhibitor1 which prevents the conversion of the androgen sex hormone testosterone into the more potent metabolite dihydrotestosterone (DHT). In 2009, South Korea has been licensed dutasteride for the treatment of androgenetic alopecia and in Japan 2015.
Dutasteride is the first and only double 5α reductase inhibitor used to treat Benign prostatic hyperplasia, and it is mainly used clinically to treat prostate enlargement, male-pattern hair loss, seborrheic hair loss, and hereditary hair loss.
Side effects
The main side effects are ED, decreased libido, gynecomastia, and ejaculation disorders. Long-term use (>4 years), however, did not reveal increased onset of sexual side effects. In addition, the combination of dutasteride and tamsulosin is well-tolerated and has the added advantage of rapid symptomatic relief.
Synthesis
Dutasteride can be prepared from 3-oxo-4-androstene-17β-carboxylic acid by several ways in 6 or 8 steps. In the preparation of dutasteride, the introduction of the carbon-carbon double bond in conjugation with C-3 carbonyl carbon of azaandrosteriods is one of the most important chemical reaction.
an efficient synthesis of dutasteride: utilizing benzoyl group as novel lactamic protecting group
Mode of action
The human body contains type I and type II 5α reductase, with type II found mainly in the prostate, and type I found mainly in the liver and skin. 5α reductase is the main cause for continuous benign prostate enlargement; it promotes the transformation of testosterone in patients’ prostate into the more active dihydrotestosterone, thus causing prostate cells to enlarge and the prostate to swell. Dutasteride can inhibit both type I and II 5α reductase at the same time. This type of simultaneous inhibiting mechanism can rapidly and continuously reduce prostate size, dramatically improve urination, and reduce the risk fo acute urinary retention and its related prostate surgeries.
Clinical claims and research
The American FDA approved a 2-year multicenter randomized double-blind control clinical trial – the first long term clinical assessment of the combined usage of Dutasteride and α receptor blockers. Included subjects were male patients with moderate to severe prostate enlargement (ages greater than or equal to 50, prostate volume (PV) ≥30 cc, serum prostate specific antigen (PSA) levels 1.5-10ng/ml, 5ml/sec < maximum urinary flow (Qmax) ≤15ml/sec, minimum urination ≥ 125ml, international prostate symptom score (IPSS) ≥ 12). Patients were first given a placebo for 4 weeks and then were randomly given either 0.5mg/day of Dutasteride and 0.4mg/day of Tamsulosin, only 0.5mg/day of Dutasteride, or only 0.4mg/day of Tamsulosin.
Results showed: After 12-24 months, the combined usage of Dutasteride with Tamsulosin had better curative effects than did individual usage.
Dutasteride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| PANCHSHEEL ORGANICS LTD | +91-9821053955 +91-9324201019 | Mumbai, India | 82 | 58 | Inquiry |
| Aurobindo Pharma Limited | +914066725000 | Telangana, India | 112 | 58 | Inquiry |
| Cipla Ltd | +912224826000 | Maharashtra, India | 133 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Horster Biotek Pvt Ltd | +91-7359657360 +91-9898127219 | Gujarat, India | 58 | 58 | Inquiry |
| Dayaram Pharma Chem | +91-9601766800 +91-9601766800 | Gujarat, India | 61 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| ANWITA APIS | 58 |
| Dr. Reddy's Laboratories Ltd | 58 |
| Gonane Pharma | 58 |
| PANCHSHEEL ORGANICS LTD | 58 |
| Aurobindo Pharma Limited | 58 |
| Cipla Ltd | 58 |
| Hetero Drugs Limited | 58 |
| Horster Biotek Pvt Ltd | 58 |
| Dayaram Pharma Chem | 58 |
Related articles
- Dutasteride: Recent advances and clinical applications in the treatment of prostate diseases and alopecia
- The purpose of this paper is to comprehensively review dutasteride's chemical properties, pharmacological effects, clinical ap....
- Sep 18,2024
164656-23-9(Dutasteride)Related Search:
1of4
chevron_right




