ARSENIC PENTASULPHIDE
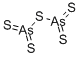
- CAS No.
- 1303-34-0
- Chemical Name:
- ARSENIC PENTASULPHIDE
- Synonyms
- As2S3;ARSENIC(V) SULFIDE;Arsenicpentasulfide;ARSENIC PENTASULPHIDE;diarsenicpentasulphide;Diarsenic pentasulfide;Arsenic sulfide (as2S5);ARSENIC(V) SULFIDE, 99.99+%
- CBNumber:
- CB3258318
- Molecular Formula:
- As2S5
- Molecular Weight:
- 310.17
- MOL File:
- 1303-34-0.mol
- Modify Date:
- 2023/10/17 17:11:53
| Melting point | >300 °C (lit.) |
|---|---|
| solubility | insoluble in H2O; soluble in alkaline solutions |
| form | brown-yellow amorphous solid |
| color | brown-yellow amorphous solid |
| Water Solubility | g/L solution H2O: 0.00136 (0°C) [KRU93] |
| Merck | 13,807 |
| CAS DataBase Reference | 1303-34-0 |
| EPA Substance Registry System | Arsenic pentasulfide (1303-34-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301+H331-H410 |
| Precautionary statements | P261-P273-P301+P310+P330-P304+P340+P312-P391-P403+P233 |
| Hazard Codes | T,N |
| Risk Statements | 23/25-50/53 |
| Safety Statements | 20/21-28-45-60-61 |
| RIDADR | UN 1557 6.1/PG 2 |
| WGK Germany | 3 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
ARSENIC PENTASULPHIDE Chemical Properties,Uses,Production
Chemical Properties
ARSENIC PENTASULPHIDE is yellow or orange powder(s); stable in air up to 95°C; can be prepared by fusing As and S or by passing H2S through HCl solution of arsenic acid; used as a paint pigment, in light filters [HAW93] [KIR78]
Physical properties
Yellow-brown glassy amorphous solid; sublimes on heating; decomposes around 500°C; insoluble in cold water (~1.4 mg/L at 0°C); dissolves in alkalies and solutions of alkali metal sulfides, and in nitric acid.
Uses
ARSENIC PENTASULPHIDE is used for preparation of glasses with small gold additions posessing photoluminescent and conductive properties 1 Reactant for preparation of silver thioarsenate complex ethylenediammonium salt 2
Preparation
Arsenic pentasulfide is prepared by precipitation from an acidic solution of orthoarsenic acid, H3AsO4, or arsenic pentachloride, AsCl5 or any other soluble As(V) salt by passing hydrogen sulfide. It may be also prepared by heating a mixture of arsenic and sulfur, extracting the fused mass with ammonia solution and reprecipitating arsenic pentasulfide at low temperature by addition of HCl.
Production Methods
Arsenic(V) sulfide, As4S10, is produced by fusing stoichiometric quantities of As and S powder or by precipitation from highly acidic arsenate(V) solution with H2S.
ARSENIC PENTASULPHIDE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| Shandong Xiya Chemical Co., Ltd. | 4009903999 13395398332 | China | 20807 | 60 | Inquiry |
| Hebei Zhenjia new material Co., LTD | 0319-5925599 13315915972 | China | 2917 | 58 | Inquiry |
| Baoji Didu Pharmaceutical and Chemical Co., Ltd | 029-81148929 13186179623 | China | 10011 | 58 | Inquiry |
| SIGMA-RBI | 800 736 3690 (Orders) | Switzerland | 6913 | 91 | Inquiry |
| Shanghai Hanhong Scientific Co.,Ltd. | 021-54306202 13764082696 | China | 42959 | 64 | Inquiry |
| Riedel-de Haen AG | 800 558-9160 | United States | 6825 | 87 | Inquiry |
| American Custom Chemicals Corporation | 858 201 6118 | United States | 6826 | 51 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |





