COPPER FORMATE
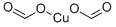
- CAS No.
- 544-19-4
- Chemical Name:
- COPPER FORMATE
- Synonyms
- COPPER FORMATE;CUPRIC FORMATE;cupricdiformate;copper diformate;COPPER (II) FORMATE;formicacid,copper(2+)salt;formicacid,copper(2++)salt;Kupfer(II)-formiat-4-hydrat;formicacid,copper(2+)salt(1:1);COPPER FORMATE ISO 9001:2015 REACH
- CBNumber:
- CB3337420
- Molecular Formula:
- C2H2CuO4
- Molecular Weight:
- 153.58
- MOL File:
- 544-19-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 130°C |
|---|---|
| Density | 1,831 g/cm3 |
| solubility | insoluble in organic solvents |
| form | Powder |
| color | green to blue |
| Specific Gravity | 1.831 |
| Water Solubility | 12.5g/100mL H2O [CRC10]; insoluble most organic solvents [MER06] |
| Merck | 14,2639 |
| EPA Substance Registry System | Formic acid, copper(2+) salt (2:1) (544-19-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS09,GHS05,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H410-H317-H301-H318 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P273-P391-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P264-P280-P302+P352-P321-P332+P313-P362-P280-P305+P351+P338-P310 |
| Hazard Codes | Xn,N |
| Risk Statements | 20/21/22-50/53-22 |
| Safety Statements | 22-36/37/39-61-60 |
| TSCA | Yes |
| Hazardous Substances Data | 544-19-4(Hazardous Substances Data) |
COPPER FORMATE Chemical Properties,Uses,Production
Chemical Properties
three forms of anhydrous formate exist: powder(s) blue, turquoise or royal blue crystal(s) [MER06].
Copper(II) formate is a royal blue material that obtains by crystallization from 75-85°C solutions. Crystallization from solutions at temperatures of 50-60°C results in the formation of a metastable dihydrate. A tetrahydrate can be produced by crystallization at lower temperatures.

Uses
Copper formate is used for the control of bacteria and mildew in cellulosic materials.
Preparation
Copper(II) formate is produced by dissolution of copper(II) oxide in hot formic acid or by the reaction of copper(II) carbonate or hydroxide with formic acid. It can also be produced by aeration of hot formic acid over copper metal.
General Description
Blue crystalline powder. Sinks and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as COPPER FORMATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
INHALATION: May cause nasal congestion. EYES: May cause conjunctivitis. SKIN: Irritation. INGESTION: Irritation.
COPPER FORMATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | China | 3692 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21628 | 55 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30231 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 7859 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 | China | 52849 | 58 | Inquiry |
| Henan Alfa Chemical Co., Ltd | +8615838112936 | China | 13194 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; | China | 35425 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | China | 26362 | 58 | Inquiry |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | China | 7245 | 58 | Inquiry |





