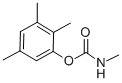
2,3,5-TRIMETHACARB
- CAS No.
- 12407-86-2
- Chemical Name:
- 2,3,5-TRIMETHACARB
- Synonyms
- broot;uc27867;LANDRIN(R);rimethacarb;trimethacarb;2,3,5-TRIMETHACARB;trimethylphenylmethylcarbamate;2,3,5-TRIMETHYLPHENYL METHYL CARBAMATE;2,3,5(or3,4,5)-trimethyl-phenomethylcarbamate;Methylcarbamic acid 2(or 4),3,5-trimethylphenyl ester
- CBNumber:
- CB3425785
- Molecular Formula:
- C11H15NO2
- Molecular Weight:
- 193.24
- MOL File:
- 12407-86-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/4 17:34:32
| Melting point | 118-123°C |
|---|---|
| Boiling point | 329.46°C (rough estimate) |
| Density | 1.0945 (rough estimate) |
| vapor pressure | 6.8 x 10-3 Pa (25 °C) |
| refractive index | 1.5080 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| Water Solubility | >58 mg l-1 (23 °C) |
| color | White to Off-White |
| EPA Substance Registry System | Trimethacarb (12407-86-2) |
SAFETY
Risk and Safety Statements
| RIDADR | 2757 |
|---|---|
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Toxicity | rabbit,LD50,skin,> 2500mg/kg (2500mg/kg),Shell Chemical Company. Vol. CODE, |
2,3,5-TRIMETHACARB Chemical Properties,Uses,Production
Uses
Trimethacarb is an insecticide with primarily stomach action. It is mainly used to control corn root worm larvae in maize but it also controls a wide range of insect and mollusc pests. Trimethacarb acts as a bird and mammal repellent.
Metabolic pathway
The metabolic pathways of trimethacarb in plants, mammals and insects involve mainly oxidation of N-methyl or ring-methyl substituents and conjugation. Some hydroxylation of the phenyl ring and ester hydrolysis occurs. The metabolism of trimethacarb was reviewed by Fukuto (1972), Kuhr and Dorough (1976) and by Schlagbauer and Schlagbauer (1972).
Degradation
Trimethacarb is hydrolysed by strong acids and alkalis but aqueous solutions are stable to light (PM). Trimethacarb was applied to bean leaves and exposed to natural sunlight for 4 days. Both isomers were decomposed more rapidly when exposed to sunlight compared with normal light in the laboratory. The compounds were almost completely decomposed. Major photoproducts resulted from hydroxylation of methyl substituents of the phenyl ring (2 and 3) and of the N-methyl group (5 and 6) (see Schemes 1 and 2). There were few products of hydrolysis (Slade and Casida, 1970; Kuhr and Dorough, 1976).
2,3,5-TRIMETHACARB Preparation Products And Raw materials
Raw materials
Preparation Products
2,3,5-TRIMETHACARB Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Alfa Chemistry | United States | 24072 | 58 | Inquiry | |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | China | 23053 | 58 | Inquiry |
| Alfa Chemistry | 1-516-6625404 | United States | 9171 | 60 | Inquiry |
| Nantong Zhonghe Chemical New Materials Co., Ltd | 13003551299 13003551299 | China | 7567 | 58 | Inquiry |
| SKC Inc. | 724-941-9701 | United States | 1379 | 76 | Inquiry |
| Riedel-de Haen AG | 800 558-9160 | United States | 6825 | 87 | Inquiry |
| Interchim S.A. | (33) 4 70 03 88 55 | France | 6307 | 76 | Inquiry |
| Dr. Ehrenstorfer GmbH | (0821) 90 60 80 | Germany | 5547 | 66 | Inquiry |
| AccuStandard Inc | (800) 442-5290 | United States | 5300 | 76 | Inquiry |
| Supplier | Advantage |
|---|---|
| Chongqing Chemdad Co., Ltd | 58 |
| Alfa Chemistry | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | 58 |
| Alfa Chemistry | 60 |
| Nantong Zhonghe Chemical New Materials Co., Ltd | 58 |
| SKC Inc. | 76 |
| Riedel-de Haen AG | 87 |
| Interchim S.A. | 76 |
| Dr. Ehrenstorfer GmbH | 66 |
| AccuStandard Inc | 76 |





