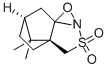
(1S)-(+)-(Camphorylsulfonyl)oxaziridine
- CAS No.
- 104322-63-6
- Chemical Name:
- (1S)-(+)-(Camphorylsulfonyl)oxaziridine
- Synonyms
- (S)-(+)-(Camphorsulfonyl)Oxazi;(+)-(Camphorsulfonyl)oxaziridine;S-(10-Camphorsulfonyl)oxaziridine;(S)-(+)-(CAMPHORSULFONYL)OXAZIRIDINE;(S)-2,N-EPOXY-EXO-10,2-BORNANESULTAM;(+)-((CAMPHORYL)SULFONYL)OXAZIRIDINE;(S)-(+)-(Camphorylsulfonyl)oxaziridine;(1S)-(+)-(CAMPHORYLSULFONYL)OXAZIRIDINE;(1S)-(+)-(10-CAMPHORSULFONYL) OXAZIRINE;(1S)-(+)-(1-Camphorsulfonyl)oxaziridine
- CBNumber:
- CB3447891
- Molecular Formula:
- C10H15NO3S
- Molecular Weight:
- 229.3
- MOL File:
- 104322-63-6.mol
- Modify Date:
- 2024/8/30 21:22:42
| Melting point | 172-174 °C(lit.) |
|---|---|
| alpha | 45 º (c=2, chloroform) |
| Boiling point | 317.6±25.0 °C(Predicted) |
| Density | 1.2486 (rough estimate) |
| refractive index | 1.5060 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| pka | -11.75±0.40(Predicted) |
| form | Crystals or Crystalline Powder |
| color | White |
| optical activity | [α]28/D +45°, c = 2 in chloroform |
| BRN | 6274370 |
| CAS DataBase Reference | 104322-63-6(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10 | |||||||||
| HS Code | 29309090 | |||||||||
| NFPA 704 |
|
(1S)-(+)-(Camphorylsulfonyl)oxaziridine price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 345350 | (1S)-(+)-(10-Camphorsulfonyl)oxaziridine | 104322-63-6 | 1G | ₹13671.98 | 2022-06-14 | Buy |
| TCI Chemicals (India) | C1326 | (2R,8aS)-(+)-(Camphorylsulfonyl)oxaziridine [Asymmetric Oxidizing Reagent] | 104322-63-6 | 1G | ₹3900 | 2022-05-26 | Buy |
| TCI Chemicals (India) | C1326 | (2R,8aS)-(+)-(Camphorylsulfonyl)oxaziridine [Asymmetric Oxidizing Reagent] | 104322-63-6 | 5G | ₹8800 | 2022-05-26 | Buy |
(1S)-(+)-(Camphorylsulfonyl)oxaziridine Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 262)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Reax Chemicals | 08048372701Ext 416 | Hyderabad, India | 4223 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Souvenier Chemicals | 08048372762Ext 645 | Mumbai, India | 396 | 58 | Inquiry |
| Changzhou Chontech Baocheng Chemical Co.,Ltd | +86-51982698291; +undefined15295095616 | China | 73 | 58 | Inquiry |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | China | 10991 | 58 | Inquiry |
(1S)-(+)-(Camphorylsulfonyl)oxaziridine Spectrum
(1S)-(+)-(Camphorylsulfonyl)oxaziridine(104322-63-6)MS(1S)-(+)-(Camphorylsulfonyl)oxaziridine(104322-63-6)1HNMR(1S)-(+)-(Camphorylsulfonyl)oxaziridine(104322-63-6)13CNMR(1S)-(+)-(Camphorylsulfonyl)oxaziridine(104322-63-6)IR1(1S)-(+)-(Camphorylsulfonyl)oxaziridine(104322-63-6)IR2(1S)-(+)-(Camphorylsulfonyl)oxaziridine(104322-63-6)Raman
104322-63-6((1S)-(+)-(Camphorylsulfonyl)oxaziridine)Related Search:
(+)-(2R,8AS)-(CAMPHORYLSULFONYL)OXAZIRIDINE
(2R,8AS)-(+)-(CAMPHORYLSULFONYL)OXAZIRIDINE
(1S)-(+)-2,N-EPOXY-EXO-10,2-BORNANESULTAM
(IS)-(+)-(10-Camphorsulfonyl)oxaziridine
S-(10-Camphorsulfonyl)oxaziridine
(4aS,7R,8aS)-9,9-DiMethyltetrahydro-4H-4a,7-Methanobenzo[c][1,2]oxazireno[2,3-b]isothiazole 3,3-dioxide
(+)-(2R,8aS)-10-(CaMphorylsulfonyl)oxaziridine
(4aS,7R,8aS)-Tetrahydro-9,9-diMethyl-4H-4a,7-Methanooxazirino[3,2-i][2,1]benzisothiazole 3,3-Dioxide
(1S)-(+)-(Camphorylsulfonyl)oxaziridine, (1S)-(+)-2,N-Epoxy-exo-10,2-bornanesultam
(1α,5β,7α)-10,10-Dimethyl-4,5-epoxy-3-thia-4-azatricyclo[5.2.1.01,5]decane3,3-dioxide
(3aS)-9,9-Dimethyloctahydro-3aβ,6β-methano-1,7aα-epoxy-2,1-benzisothiazole 2,2-dioxide
(4aS,7R,8aS)-5,6,7,8-Tetrahydro-9,9-dimethyl-4H-4a,7-methanooxazirino[3,2-i][2,1]benzoisothiazole 3,3-dioxide
(4aS,7R,8aS)-9,9-Dimethyl-4H-4a,7-methanotetrahydrooxazirino[3,2-i][2,1]benzisothiazole 3,3-dioxide
(8aS)-5,6,7,8-Tetrahydro-9,9-dimethyl-4H-4aβ,7β-methanooxazirino[3,2-i][2,1]benzisothiazole 3,3-dioxide
(+)-(2R,8aS)-(Camphorylsulfonyl)oxaziridine,98+%
(4AS,7R,8aS)-9,9-Dimethyltetrahydro-4H-4a,7-methanobenzo-[c][1,2]oxazireno[2,3-b]isothiazole 3,3-
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine
(1S)-(+)-(Camphorylsulfonyl)oxaziridine
(S)-(+)-(Camphorylsulfonyl)oxaziridine
(4AS,7R,8aS)-9,9-Dimethyltetrahydro-4H-4a,7-methanobenzo-[c][1,2]oxazireno[2,3-b]isothiazole 3
(1S)-(+)-(CAMPHORYLSULFONYL)OXAZIRIDINE
(1S)-(+)-(10-CAMPHORSULFONYL)OXAZIRIDINE
(1S)-(+)-(10-CAMPHORSULPHONYL)OXAZIRIDINE
(S)-(+)-(CAMPHORSULFONYL)OXAZIRIDINE
(S)-2,N-EPOXY-EXO-10,2-BORNANESULTAM
(+)-((CAMPHORYL)SULFONYL)OXAZIRIDINE
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine (1S)-(+)-2,N-Epoxy-exo-10,2-bornanesultam
(+)-(2R,8aS)-(Camphorylsulfonyl)oxaziridine, 99+% ee, 99+%
(S)-(+)-(Camphorsulfonyl)Oxazi
(1S)-(+)-(10-CAMPHORSULFONYL) OXAZIRINE
(2R 8AS)-(+)-(CAMPHORYLSULFONYL)OXAZIRIDINE [ASYMMETRIC OXIDIZING GR]
(2R,8aS)-(+)-(Camphorylsulfonyl)oxaziridine [Asymmetric Oxidizing Reagent]
(S)-(10-Camphorsulfonyl)oxaziridine,99%e.e.
(+)-(2R, 8aS)-(Camphorylsulfonyl)oxaziridine, 99+%, (99+% e.e.)
(1S)-(+)-(1-Camphorsulfonyl)oxaziridine
(2R,8aS)-(+)-(Camphorylsulfonyl)oxaziridine[AsymmetricOxidizingReagent]>
4H-4a,7-Methanooxazirino[3,2-i][2,1]benzisothiazole, tetrahydro-9,9-dimethyl-, 3,3-dioxide, (4aS,7R,8aS)-
CAS104322-63-6 (1S)-(+)-(Camphorylsulfonyl)oxaziridine
(+)-(2R,8aS)-(Camphorylsulfonyl)oxaziridine
(+)-(Camphorsulfonyl)oxaziridine
(4aS,7S,8aS)-9,9-dimethyltetrahydro-4H-4a,7-methanobenzo[c][1,2]oxazireno[2,3-b]isothiazole 3,3-dioxide
(1S,8R)-11,11-dimethyl-5-oxa-3λ?-thia-4-azatetracyclo[6.2.1.0?,?.0?,?]undecane-3,3-dione
(1S,8R)-11,11-dimethyl-5-oxa-3λ?-thia-4-azatetracyclo[6.2.1.01,?.0?,?]undecane-3,3-dione
104322-63-6
C10H15NO3S
Hydroxylation
Asymmetric Synthesis
Sulfur Compounds (for Synthesis)
Bicyclic Monoterpenes
Oxidation
Terpenes
Chiral Catalysts, Ligands, and Reagents
Intermediates & Fine Chemicals
Pharmaceuticals
Chiral Reagents
pharmacetical
chiral
Asymmetric Synthesis
Bicyclic Monoterpenes





