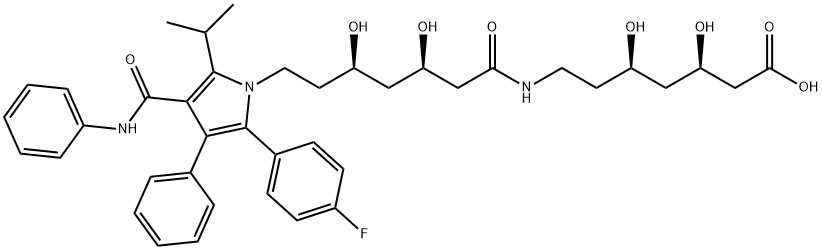
Atorvastatin EP IMpurity F
- CAS No.
- 887196-24-9
- Chemical Name:
- Atorvastatin EP IMpurity F
- Synonyms
- ATV imp F;AtorvastatinEPImpurity-F;Atorvastatin Amide Impurity;Atorvastatin EP Impurity F Sodium Salt;Atorvastatin Calcium Hydrate impurity F;Atorvastatin IMpurity F (AMide IMpurity) SodiuM Salt;(3R,5R)-7-((3R,5R)-7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanamido)-3,5-dihydroxyheptanoic aci;(3R,5R)-7-[[(3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoyl]amino]-3,5-dihydroxyheptanoic acid;Heptanoic acid, 7-[[(3R,5R)-7-[2-(4-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrol-1-yl]-3,5-dihydroxy-1-oxoheptyl]amino]-3,5-dihydroxy-, (3R,5R)-;Atorvastatin EP Impurity F (3R,5R)-7-[[(3R,5R)-7-[2-(4-fluorophenyl)-5-(1-methylethyl) -3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5- dihydroxyheptanoyl]amino]-3,5-dihydroxyheptanoic acid
- CBNumber:
- CB42673529
- Molecular Formula:
- C40H48FN3O8
- Molecular Weight:
- 717.82
- MOL File:
- 887196-24-9.mol
- Modify Date:
- 2022/12/21 16:56:50
Atorvastatin EP IMpurity F Preparation Products And Raw materials
Atorvastatin EP IMpurity F Suppliers
Global( 37)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BTC pharm India | +918790379245 | AndhraPradesh, India | 1269 | 58 | Inquiry |
| CLEANCHEM LABORATORIES LLP | +91-9951015911 +91-9951015911 | Mumbai, India | 281 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 461 | 58 | Inquiry |
| SynThink RESEARCH CHEMICALS | +91-8177860948 +91-8177860948 | Mumbai, India | 494 | 58 | Inquiry |
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt. ltd | +91 8550986868 | Maharashtra, India | 736 | 58 | Inquiry |
887196-24-9(Atorvastatin EP IMpurity F)Related Search:
Atorvastatin Impurity 27
Atorvastatin IMpurity 8
(1R,3S)-1-(1,3-Benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid Methyl Ester
Atorvastatin Related Compound C
Atorvastatin IMpurity 3
N,3-Diphenyl-2-(2-methyl-1-oxopropyl)4-oxo-N-benzenebutanamide
(Mixture of Diastereomers)
Atorvastatin Impurity 16
3S, 5S enantioMer of Atorvastatin CalciuM
Atorvastatin Impurity 22
Atorvastatin IMpurity 6
chevron_left
1of4
chevron_right
Atorvastatin IMpurity F (AMide IMpurity) SodiuM Salt
Atorvastatin EP Impurity F Sodium Salt
ATV imp F
Heptanoic acid, 7-[[(3R,5R)-7-[2-(4-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrol-1-yl]-3,5-dihydroxy-1-oxoheptyl]amino]-3,5-dihydroxy-, (3R,5R)-
(3R,5R)-7-((3R,5R)-7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanamido)-3,5-dihydroxyheptanoic aci
(3R,5R)-7-[[(3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoyl]amino]-3,5-dihydroxyheptanoic acid
Atorvastatin Calcium Hydrate impurity F
Atorvastatin Amide Impurity
Atorvastatin EP Impurity F
(3R,5R)-7-[[(3R,5R)-7-[2-(4-fluorophenyl)-5-(1-methylethyl)
-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-
dihydroxyheptanoyl]amino]-3,5-dihydroxyheptanoic acid
AtorvastatinEPImpurity-F
887196-24-9
115067-87-5
C40H48FN3O8





