ChemicalBook > Product Catalog >API >Synthetic Anti-infective Drugs >Antifungal Drugs >ANTIPAIN HYDROCHLORIDE DIHYDRATE
ANTIPAIN HYDROCHLORIDE DIHYDRATE
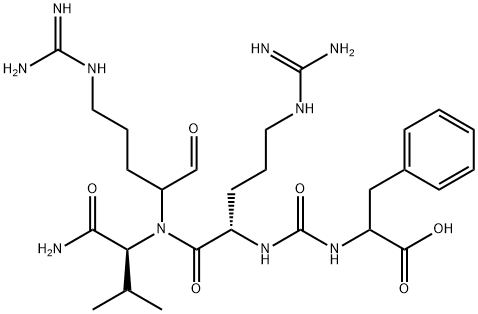
- CAS No.
- 37691-11-5
- Chemical Name:
- ANTIPAIN HYDROCHLORIDE DIHYDRATE
- Synonyms
- Aids006469;Aids-006469;PHE-ARG-VAL-ARG-CHO;ANTIPAIN ~10000 U/MG;Phe-co-Arg-Val-L-Arg-h;Antipain research grade;ANTIPAIN HYDROCHLORIDE DIHYDRATE;ANTIPAIN HYDROCHLORIDE DIHYDRATE USP/EP/BP;4-((aminoiminomethyl)amino)-1-formylbutyl)-;Nα-[Nα-[[(1-Carboxy-2-phenylethyl)amino]carbonyl]-L-arginyl]-N-(1-formyl-4-guanidinobutyl)-L-valinamide
- CBNumber:
- CB4279464
- Molecular Formula:
- C27H44N10O6
- Molecular Weight:
- 604.7
- MOL File:
- 37691-11-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/15 10:43:29
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| WGK Germany | 3 |
| RTECS | YV9350700 |
| F | 1-10 |
ANTIPAIN HYDROCHLORIDE DIHYDRATE price More Price(2)
ANTIPAIN HYDROCHLORIDE DIHYDRATE Chemical Properties,Uses,Production
General Description
Chemical structure: peptide
Biochem/physiol Actions
Reversible inhibitor of serine/cysteine proteases and some trypsin-like serine proteases. Its action resembles leupeptin; however, its plasmin inhibition is less and its cathepsin A inhibition is more than that observed with leupeptin. Chronic administration can reduce the frequency of chemically induced transformation in BALB/c-/3T3 cells.
ANTIPAIN HYDROCHLORIDE DIHYDRATE Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 73)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26165 | 58 | Inquiry |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | China | 19915 | 58 | Inquiry |
| Nanjing TGpeptide | +86-13347807150 +86-13347807150 | China | 3279 | 58 | Inquiry |
| Zhejiang Hangyu API Co., Ltd | +8617531972939 | China | 2944 | 58 | Inquiry |
| Shanghai Maclean Biochemical Technology Co., LTD | 021-50706066 15221275939 | China | 29803 | 58 | Inquiry |
| Baoji Didu Pharmaceutical and Chemical Co., Ltd | 029-81148929 13186179623 | China | 10011 | 58 | Inquiry |
| Zhejiang Jiekun Biotechnology Co., Ltd | 0571-88211951 13735575465 | China | 4904 | 58 | Inquiry |
| Chengdu Youngshe Chemical Co., Ltd. | +86-17380623303 +86-17380623303 | China | 5948 | 58 | Inquiry |
37691-11-5(ANTIPAIN HYDROCHLORIDE DIHYDRATE)Related Search:
N2-[[(1-Carboxy-2-phenylethyl)amino]carbonyl]-L-arginyl-N-[4-[(aminoiminomethyl)amino]-1-formylbutyl]-L-valinamide
PHE-ARG-VAL-ARG-CHO
Antipain research grade
4-((aminoiminomethyl)amino)-1-formylbutyl)-
ANTIPAIN HYDROCHLORIDE DIHYDRATE
N2-[[(1-carboxyphenethyl)amino]carbonyl]-L-arginyl-N-[4-[(aminoiminomethyl)amino]-1-formylbutyl]-L-valinamide
ANTIPAIN ~10000 U/MG
Nα-[N2-[[(1-Carboxy-2-phenylethyl)amino]carbonyl]-L-arginyl]-N-[4-[(aminoiminomethyl)amino]-1-formylbutyl]-L-valinamide
Nα-[Nα-[[(1-Carboxy-2-phenylethyl)amino]carbonyl]-L-arginyl]-N-(1-formyl-4-guanidinobutyl)-L-valinamide
Aids006469
Aids-006469
L-Valinamide, N(sup 2)-(((1-carboxy-2-phenylethyl)amino)carbonyl)-L-arginyl-N-(4-((aminoiminomethyl)amino)-1-formylbutyl)-
Phe-co-Arg-Val-L-Arg-h
L-Valinamide, N2-[[(1-carboxy-2-phenylethyl)amino]carbonyl]-L-arginyl-N-[4-[(aminoiminomethyl)amino]-1-formylbutyl]-
ANTIPAIN HYDROCHLORIDE DIHYDRATE USP/EP/BP
37691-11-5
C27H44N10O6HCl
C27H49ClN10O8
C27H44N10O6
A - K
Aldehydes
Antibacterial
Antibiotics A to Z
Antibiotics
Antibiotics A-F
Antibiotics by Application
Antineoplastic and Immunosuppressive Antibiotics
Building Blocks
C13-C60
Carbonyl Compounds
Chemical Structure Class
Chemical Synthesis
Inhibits an Enzyme
Mechanism of Action
Organic Building Blocks
Peptides
Spectrum of Activity
Pepetides





