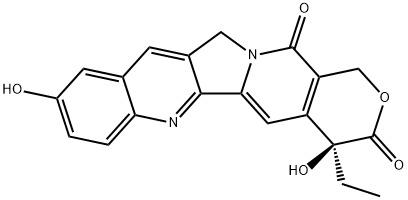
10-Hydroxycamptothecin
- CAS No.
- 19685-09-7
- Chemical Name:
- 10-Hydroxycamptothecin
- Synonyms
- SN38;HCPT;HYDROXYCAMPTOTHECIN;10-HCPT;(S)-10-HYDROXYCAMPTOTHECIN;Ampule;NSC 107124;HYDROCAMPTOTHECINE;hydroxy-camptotheci;Irinotecan USP RC A
- CBNumber:
- CB4445430
- Molecular Formula:
- C20H16N2O5
- Molecular Weight:
- 364.35
- MOL File:
- 19685-09-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/8 9:02:14
| Melting point | 265-270°C |
|---|---|
| Boiling point | 820.7±65.0 °C(Predicted) |
| Density | 1.60 |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≥23.8 mg/mL in DMSO with gentle warming; insoluble in EtOH; insoluble in H2O |
| form | powder to crystal |
| pka | 8.93±0.40(Predicted) |
| color | White to Yellow to Orange |
| InChIKey | HAWSQZCWOQZXHI-FQEVSTJZSA-N |
| CAS DataBase Reference | 19685-09-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H340 | |||||||||
| Precautionary statements | P202-P264-P270-P280-P301+P310-P405 | |||||||||
| Safety Statements | 24/25 | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
10-Hydroxycamptothecin price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR2491 | Irinotecan Related Compound A certified reference material, pharmaceutical secondary standard | 19685-09-7 | 25MG | ₹44804.68 | 2022-06-14 | Buy |
| TCI Chemicals (India) | H1463 | 10-Hydroxycamptothecin | 19685-09-7 | 1G | ₹14200 | 2022-05-26 | Buy |
10-Hydroxycamptothecin Chemical Properties,Uses,Production
Description
DNA topoisomerases relax supercoiled DNA during replication, transcription, recombination, repair, and chromosome condensation. The relaxation of DNA supercoiling by topoisomerase I at single-
Chemical Properties
Yellow Solid
Uses
A Camptothecin derivative; a topoisomerase inhibitor for cancer therapy
10-Hydroxycamptothecin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| Avra Laboratories Pvt Ltd | +91-8008572391 +91-8008572391 | Hyderabad, India | 43 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| AVRA LABORATORIES PRIVATE LTD | +91-040- 27178571 | New Delhi, India | 3 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Manus Aktteva | +91 (79) 6512-3395 | New Delhi, India | 581 | 34 | Inquiry |
| VIVAN Life Sciences Pvt. Limited. | +91(22) 2544 4584 / 2544 4585 | New Delhi, India | 138 | 50 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
19685-09-7(10-Hydroxycamptothecin)Related Search:
1of4
chevron_right




