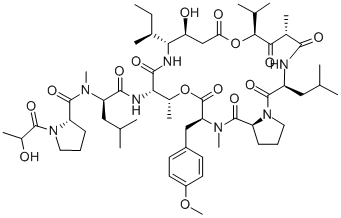
Didemnin B
- CAS No.
- 77327-05-0
- Chemical Name:
- Didemnin B
- Synonyms
- Didemnin B;Didemnin A, N-[1-[(2S)-2-hydroxy-1-oxopropyl]-L-prolyl]-;N-(L-Lac-L-Pro-N-Methyl-D-Leu-)cyclo[L-Thr*-[(3S,4R)-3-hydroxy-4-[(S)-1-methylpropyl]-γAbu-]-[(2S,4S)-4-hydroxy*-2,5-dimethyl-3-oxohexanoyl]-L-Leu-L-Pro-N,O-dimethyl-L-Tyr-]
- CBNumber:
- CB51247302
- Molecular Formula:
- C57H89N7O15
- Molecular Weight:
- 1112.36
- MOL File:
- 77327-05-0.mol
- Modify Date:
- 2025/1/27 9:38:02
| Boiling point | 821.94°C (rough estimate) |
|---|---|
| Density | 1.0596 (rough estimate) |
| refractive index | 1.6910 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Acetonitrile: 1 mg/ml |
| form | A crystalline solid |
| pka | 11.28±0.70(Predicted) |
| color | White to off-white |
| optical activity | -82.0622 (CHCl3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H400-H410 | |||||||||
| Precautionary statements | P264-P273-P280-P305+P351+P338-P337+P313-P391-P501 | |||||||||
| NFPA 704 |
|
Didemnin B Chemical Properties,Uses,Production
Description
Didemnin B is a cyclic depsipeptide extracted from the Caribbean tunicate Trididemnum cyanophorum. Didemnin B activates caspase, inducing apoptosis and preventing eukaryotic elongation factor 2 (eEF-2)-dependent translocation, inhibiting protein synthesis. This agent also has immunosuppressive and antiviral properties. It is an effective immunosuppressive agent. It is approximately 1,000 times more effective than the standard cyclosporin A. Further study may prove it useful in repressing rejections of transplanted organs[1].
Definition
ChEBI: A natural product found particularly in Lyngbya majuscula and Trididemnum solidum.
Biological Activity
Didemnin B belongs to a group of cyclic depsipeptides produced by Trididemnum solidum Van Name and provides a unique lactylprolin unit in the side chain of the molecule. The natural product interacts with the 80S ribosome, thereby retaining eEF1α in the ribosomal complex despite exhibiting proper GTP hydrolysis. Consequently, eEF2 binding to the ribosome and subsequent eEF2-dependent tRNA translocation are prevented. Didemnin B only shows sufficient binding affinity to the ribosome-eEF1α-complex and not to eEF1α alone. The natural product has antiviral properties and is cytotoxic against both cancerous and normal mammalian cells, whereby highly proliferating cells are more strongly affected than resting cells[2].
in vitro
Didemnin B has activity against miirine B16 melanoma, P388 leukemia, and M5076 sarcoma.
in vivo
Didemnin B is a potent inhibitor of L1210 growth.
References
[1] Renneberg, R. Viola Berkling and V. Loroch. “Chapter 7 – Green Biotechnology.”Biotechnology for Beginners (Third Edition) (2023): 241-290.
[2] Luisa D. Burgers , Robert Fürst. “Natural products as drugs and tools for influencing core processes of eukaryotic mRNA translation.” Pharmacological research 170 (2021): Article 105535.
Didemnin B Preparation Products And Raw materials
Raw materials
Preparation Products
Didemnin B Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Shanghai Minbiotech Co., Ltd. | +8617315815539 | CHINA | 129 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21628 | 55 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19552 | 58 | Inquiry |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | China | 5738 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32202 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15393 | 58 | Inquiry |
| Henan Alfa Chemical Co., Ltd | +8615838112936 | China | 13194 | 58 | Inquiry |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | China | 3135 | 58 | Inquiry |
| Aladdin Scientific | United States | 52924 | 58 | Inquiry |
77327-05-0(Didemnin B)Related Search:
1of3
chevron_right




