[[difluoro(triMethylsilyl)Methyl]thio]-Benzene
![[[difluoro(triMethylsilyl)Methyl]thio]-Benzene Structure](CAS/GIF/536975-49-2.gif)
- CAS No.
- 536975-49-2
- Chemical Name:
- [[difluoro(triMethylsilyl)Methyl]thio]-Benzene
- Synonyms
- [Difluoro(phenylthio)Methyl]triMethylsilane;[[difluoro(triMethylsilyl)Methyl]thio]-Benzene;[Difluoro(phenylsulfanyl)methyl]trimethylsilane;Benzene, [[difluoro(trimethylsilyl)methyl]thio]-
- CBNumber:
- CB52644988
- Molecular Formula:
- C10H14F2SSi
- Molecular Weight:
- 232.37
- MOL File:
- 536975-49-2.mol
- Modify Date:
- 2024/1/25 15:46:45
[[difluoro(triMethylsilyl)Methyl]thio]-Benzene Chemical Properties,Uses,Production
Description
An effective reagent to introduce difluoromethyl groups into carbonyls, imines, enamines, and alkyl halides. Not only various simple aldehydes and ketones, but also functionalized carbonyls such as α- and γ-ketoesters and cyclic imides can be difluoro(phenylthio)methylated in high yields under the activation of a catalytic amount of Lewis bases. The substitution reaction proceeds well with primary alkyl bromides and iodides as the limiting reactant when cesium fluorode/15-crown-5 is used as the fluoride source/additive. Under radical conditions, the difluoro(phenylthio)methyl compounds containing vinyl functional groups can form 5- or 6-membered rings via intramolecular cyclization.
Physical properties
colorless liquid; bp 86–87?C/4 mmHg.
Uses
Organofluorine compounds have received remarkable
interest in recent years due to their wide-ranging biological
effects. The development of general synthetic routes to such compounds
and the use of new fluorinated compounds as building
blocks are of great importance. Of particular interest is the selective
incorporation of the gem-difluoromethylene group ‘CF2’ into
organic molecules. The report on the synthesis of PhSCF2SiMe3
was published in 2003 by Prakash et al., and the reagent has
become one of the most versatile and efficient nucleophilic
(phenylthio)difluoromethylating reagents.
When excess potassium t-butoxide
was used as a promoter, PhSCF2SiMe3 reacted with diphenyl
disulfide to give the corresponding dithioacetal in 85%
yield (eq 8).
Preparation
[difluoro(phenylthio)methyl]trimethylsilane (PhSCF2SiMe3) was prepared for the first time by Prakash et al.,1 using the Barbier coupling reaction of bromodifluoromethylphenyl sulfide,2 prepared from dibromodifluoromethane and sodium benzenethiolate,3 magnesium metal, and chlorotrimethylsilane (TMSCl) in DMF (eq 1).
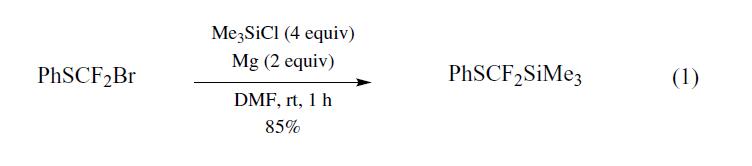
Reactions
(1) Difluoromethylation of aldehydes and ketones.
(2) Difluoromethylation of imines and enamines.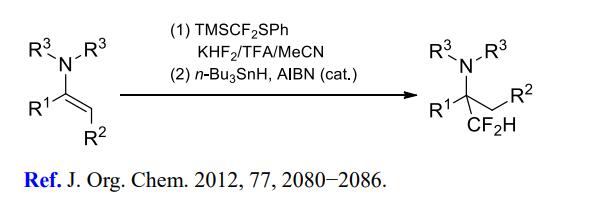
(3) (Phenylthio)difluoromethylation of imines for further cyclizations.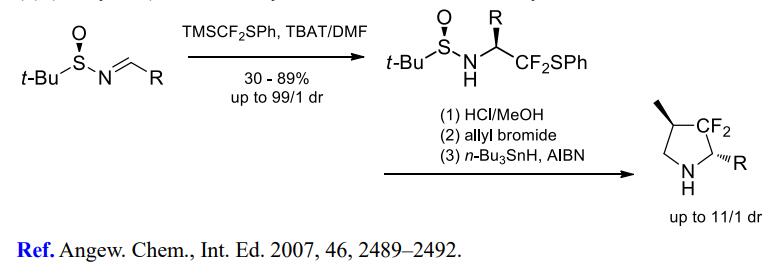
(4) Difluoromethylation of alkyl halides.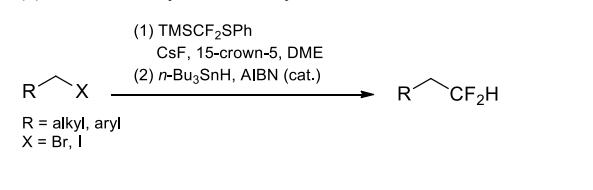
References
[1] TEERAWUT BOOTWICHA. Fluoride-Catalyzed Addition of PhSCF2SiMe3 to N-Substituted Cyclic Imides Followed by Radical Cyclization: General Synthetic Strategy of gem-Difluoromethylenated 1-Azabicyclic Compounds[J]. The Journal of Organic Chemistry, 2009, 74 10: 3798-3805. DOI:10.1021/jo802794u.
[2] MIKHAIL D. KOSOBOKOV. Reactions of Sulfur- and Phosphorus-Substituted Fluoroalkylating Silicon Reagents with Imines and Enamines under Acidic Conditions[J]. The Journal of Organic Chemistry, 2012, 77 4: 2080-2086. DOI:10.1021/jo202669w.
[3] YA LI Jinbo H Prof??Dr. Stereoselective Difluoromethylenation Using Me3SiCF2SPh: Synthesis of Chiral 2,4-Disubstituted 3,3-Difluoropyrrolidines?[J]. Angewandte Chemie International Edition, 2007, 46 14: 2489-2492. DOI:10.1002/anie.200604783.
[[difluoro(triMethylsilyl)Methyl]thio]-Benzene Preparation Products And Raw materials
[[difluoro(triMethylsilyl)Methyl]thio]-Benzene Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | China | 10991 | 58 | Inquiry |
| Shandong Mopai Biotechnology Co., Ltd | 13070690626 | China | 10785 | 58 | Inquiry |
| Daicel Chiral Technologies (China)CO.,LTD | 021-50460086-9 15921403865 | China | 7182 | 65 | Inquiry |
| Shanghai Ruipu Medical Technology Co., Ltd | 15895968936 | China | 9996 | 58 | Inquiry |
| Nantong Hanfang Biotechnology Co. , Ltd. | 18616537568 | China | 30968 | 58 | Inquiry |
| Matrix Scientific | 803 788-9494 All other calls | United States | 6655 | 80 | Inquiry |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | China | 94658 | 76 | Inquiry |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 +86-13028896684; | China | 56439 | 58 | Inquiry |
| Synthonix Inc | 001-9198759277 | United States | 6885 | 58 | Inquiry |
| Combi-Blocks Inc. | 858 635 8950 | United States | 6632 | 69 | Inquiry |





