copper acetylide
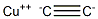
- CAS No.
- 12540-13-5
- Chemical Name:
- copper acetylide
- Synonyms
- copper acetylide;copper(ii) acetylide
- CBNumber:
- CB54646760
- Molecular Formula:
- C2Cu
- Molecular Weight:
- 87.5674
- MOL File:
- 12540-13-5.mol
- Modify Date:
- 2023/10/17 17:11:45
| Melting point | 100°C (rough estimate) |
|---|---|
| form | brown-black solid |
| color | brown-black solid; explodes, explosive |
| EPA Substance Registry System | Copper acetylide (12540-13-5) |
copper acetylide Chemical Properties,Uses,Production
Chemical Properties
brown-black solid; explodes [CRC10]
Physical properties
Brownish black powder; insoluble in water.
Uses
Cupric acetylide is used as a detonator.Its applications are very limited, however,because of its high sensitivity to impact orfriction. It is susceptible to form and buildup upon prolong contact of copper metal withorganic vapors.
Preparation
Copper(II) acetylide may be prepared by passing alkyl acetylene vapors over aqueous solution of ammoniacal copper salt.
Hazard
Copper(II) acetylide is highly sensitive to impact, friction or heat. Mild impact or heating can cause a violent explosion. In the dry state it is flammable and is more sensitive to impact or friction than copper(I) acetylide.
Fire Hazard
Cupric acetylide is much more sensitive to impact and friction than the cuprous salt. Friction heating or mild impact can result in violent explosion. In dry state, its sensitivity is much greater to impact and is flammable.
Safety Profile
Confirmed carcinogen. Cases of berylliosis have been reported from exposure to so-called low berylhum alloys. Human systemic effects by inhalation: dyspnea, fibrosing alveolitis, weight loss, or decreased weight gain. See also BERYLLIUM COMPOUNDS and COPPER COMPOUNDS. When heated to decomposition it emits very toxic fumes of BeO.





