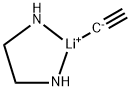
Lithium acetylide ethylenediamine complex
- CAS No.
- 6867-30-7
- Chemical Name:
- Lithium acetylide ethylenediamine complex
- Synonyms
- LITHIUM ACETYLIDE;ide, ethyL;ithium acetyL;enediamine compL;Lithium, (1,2-ethaned;1,2-Ethanediamine-ethynyllithium (1:1);LITHIUM ACETYLIDE-ETHYLENEDIAMINE COMPLE;Lithium acetylidethylenediamine complex;LITHIUM ACETYLIDE ETHYLENEDIAMINE COMPLEX;lithium acetylene, ethylenediamine complex
- CBNumber:
- CB2707213
- Molecular Formula:
- C4H7LiN2
- Molecular Weight:
- 90.05
- MOL File:
- 6867-30-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 76 °C (dec.) |
|---|---|
| Boiling point | 110.6 °C (lit.) |
| Density | 1,04 g/cm3 |
| Flash point | 7 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in ethylene diamine, n-propyl amine, n-butyl amine, pyridine, dimethyl formamaide, diglyme, dioxan and dimethylsulfoxide. |
| form | Crystals or Crystalline Powder |
| color | Slightly yellow to light brownor |
| Sensitive | Moisture Sensitive |
| BRN | 3593824 |
| InChIKey | QJQWXTYPTBEPGS-UHFFFAOYSA-N |
| CAS DataBase Reference | 6867-30-7(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium, (1,2-ethanediamine-.kappa.N,.kappa.N')ethynyl- (6867-30-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H261-H314 | |||||||||
| Precautionary statements | P231+P232-P260-P280-P301+P330+P331-P303+P361+P353-P305+P351+P338+P310 | |||||||||
| Hazard Codes | F,C | |||||||||
| Risk Statements | 15-34-67-65-63-48/20-11-35-14/15-20/21/22-14 | |||||||||
| Safety Statements | 26-43-45-62-36/37/39-43B-16-6A-7/8-3/7/9 | |||||||||
| RIDADR | UN 3399 4.3/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 38249095 | |||||||||
| NFPA 704 |
|
Lithium acetylide ethylenediamine complex price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 186155 | Lithium acetylide, ethylenediamine complex 90% | 6867-30-7 | 10G | ₹3810.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 186155 | Lithium acetylide, ethylenediamine complex 90% | 6867-30-7 | 50G | ₹10002.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 186155 | Lithium acetylide, ethylenediamine complex 90% | 6867-30-7 | 100G | ₹10597.68 | 2022-06-14 | Buy |
| ALFA India | ALF-L12401-22 | Lithium acetylide ethylenediamine complex, tech. 85% | 6867-30-7 | 100g | ₹13919 | 2022-05-26 | Buy |
| ALFA India | ALF-L12401-14 | Lithium acetylide ethylenediamine complex, tech. 85% | 6867-30-7 | 25g | ₹5259 | 2022-05-26 | Buy |
Lithium acetylide ethylenediamine complex Chemical Properties,Uses,Production
Chemical Properties
Lithium Acetylide Ethylenediamine Complex is a slightly yellow to light brown crystals, Lithium acetylide is an unstable solid which disproportionates to form lithium carbide (dilithium acetylide) and acetylene. It is soluble in liquid ammonia at its boiling point to the extent of just over one mole per liter. It is not sensitive to shock and presents a hazard due only to possible caustic burns and the hazards presented by ammonia and acetylene, which form when the material contacts the air.
Uses
Lithium acetylide ethylenediamine complex is used in the synthesis of a chiral cyclopropanol. It is also used as an alkynylating reagent for the enantioselective alkynylation of ketones catalyzed by lithium binaphtholate without using other metal sources.
Production Methods
Lithium acetylide is produced both in the laboratory and industrially by dissolving lithium metal in refluxing liquid ammonia and then bubbling acetylene into the solution. The solution of monolithium acetylide thus formed is stabilized by the complexing of the lithium ion with ammonia forming a polar acetylenic anion which does not react further with lithium metal. However, if the reaction is carried out in tetrahydrofuran, for example, the lithium acetylide reacts further with lithium to form dilithium acetylide (lithium carbide). In fact, if the ammonia is removed from a solution of lithium acetylide, it disproportionates to dilithium acetylide and acetylene.
Preparation
The ethylenediamine complex of lithium acetylide is produced by allowing lithium metal to react with ethylenediamine in benzene at reflux to form AMithioethylenediamine: H2NCH2CH2NH2+Li -> L1HNCH2CH2NH2+05H2.
Lithium acetylide ethylenediamine complex Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ALS INDIA LIFE SCIENCES | +91-8977036379 +91-8977036379 | Hyderabad, India | 1878 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Parad Chem Corporation | +91-2662 244460 | Gujarat, India | 7 | 58 | Inquiry |
| ASM Organics | 91-9866122393 | Andhra Pradesh, India | 1185 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| Avra Synthesis Pvt Ltd | +914027175796 | New Delhi, India | 1603 | 30 | Inquiry |
| Molkem Chemicals Pvt. Ltd. | 91-79-61202500 | Gujarat, India | 199 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ALS INDIA LIFE SCIENCES | 58 |
| JSK Chemicals | 58 |
| Parad Chem Corporation | 58 |
| ASM Organics | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| CHEMSWORTH | 30 |
| Alfa Aesar | 58 |
| Avra Synthesis Pvt Ltd | 30 |
| Molkem Chemicals Pvt. Ltd. | 58 |
6867-30-7(Lithium acetylide ethylenediamine complex)Related Search:
1of4
chevron_right




