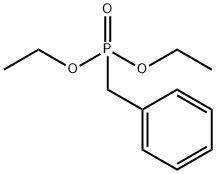
diethyl benzylphosphonate
- CAS No.
- 1080-32-6
- Chemical Name:
- diethyl benzylphosphonate
- Synonyms
- BENV-006;NSC 62294;Diethylbenzylphosphonat;DIETHYL BENZOPHOSPHONATE;DIETHYLBENZYLPHOSPHORATE;DIETHYL BENZYLPHOSPHONATE;DIETHOXY BENZYLPHOSPHONATE;Diethylα-toluenephosphonate;Diethyl phosphonate, benzyl-;diethyl à-toluenephosphonate
- CBNumber:
- CB5696730
- Molecular Formula:
- C11H17O3P
- Molecular Weight:
- 228.22
- MOL File:
- 1080-32-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/3/7 8:52:18
| Boiling point | 106-108 °C1 mm Hg(lit.) |
|---|---|
| Density | 1.095 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| form | Liquid |
| Specific Gravity | 1.10 |
| color | Clear colorless to slightly yellow |
| Water Solubility | Insoluble in water. |
| BRN | 2580931 |
| CAS DataBase Reference | 1080-32-6(CAS DataBase Reference) |
| EPA Substance Registry System | Phosphonic acid, (phenylmethyl)-, diethyl ester (1080-32-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Risk Statements | 36/38-36/37/38 | |||||||||
| Safety Statements | 24/25-37-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | SZ6600000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
diethyl benzylphosphonate price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | D91071 | Diethyl benzylphosphonate 99% | 1080-32-6 | 25G | ₹4773.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D91071 | Diethyl benzylphosphonate 99% | 1080-32-6 | 100G | ₹15349.85 | 2022-06-14 | Buy |
| TCI Chemicals (India) | B1795 | Diethyl Benzylphosphonate min. 98.0 % | 1080-32-6 | 25G | ₹2800 | 2022-05-26 | Buy |
diethyl benzylphosphonate Chemical Properties,Uses,Production
Chemical Properties
CLEAR COLOURLESS TO SLIGHTLY YELLOW LIQUID
Uses
Diethyl benzylphosphonate is used as a reactant for synthesis of 3,5-dihydroxy-4-isopropylstilbene for treatment of skin disorders and natural cytotoxic marine products of polyketide origin via intramolecular Diels-Alder reactions. It is also used as reactant for cyclization of aryl ethers, amines and amides & for investigating the effects of functional groups on the performance of clue organic LEDs.
Preparation
Diethyl benzylphosphonate was synthesized according to the previously published procedure [1]. Benzylphosphonic acid (1 mmol, 172 mg) and triethyl orthoacetate (30 mmol, 5.5 mL) were mixed overnight at 90 °C. The completion of the reaction was monitored by 31P NMR. After the completion of the reaction, an excess of the orthoester was evaporated under reduced pressure. The crude product was purified via a short silica gel column (hexanes/ethyl acetate). The compound 1 was isolated as a transparent oil with 98% yield (0.98 mmol, 224 mg). 1H NMR (400 MHz, DMSO-d6) δ 7.39–7.14 (m, 5H), 3.94 (dq, JH-P = 7.9, JH-H = 7.0, 4H), 3.21 (d, JH-P = 21.6 Hz, 2H), 1.16 (t, JH-H = 7.0 Hz, 6H); 13C NMR (101 MHz, DMSO-d6) δ 132.3 (d, JC-P = 9.0 Hz), 129.7 (d, JC-P = 6.6 Hz), 128.2 (d, JC-P = 3.0 Hz), 126.4 (d, JC-P = 3.4 Hz), 61.3 (d, JC-P = 6.5 Hz), 32.3 (d, JC-P = 135.1 Hz), 16.1 (d, JC-P = 5.8 Hz); 31P NMR (162 MHz, DMSO-d6) δ 26.5. The NMR data are in accordance with those reported in literature [2].
Synthesis Reference(s)
Tetrahedron Letters, 29, p. 1513, 1988 DOI: 10.1016/S0040-4039(00)80339-1
Synthesis, p. 222, 1981 DOI: 10.1055/s-1981-29393
References
[1] DAMIAN TRZEPIZUR. Selective Esterification of Phosphonic Acids.[J]. Molecules, 2021. DOI:10.3390/molecules26185637.
[2] PETER J. THORNTON. Nucleoside Phosphate and Phosphonate Prodrug Clinical Candidates[J]. Journal of Medicinal Chemistry, 2016, 59 23: 10400-10410. DOI:10.1021/acs.jmedchem.6b00523.
[3] GASTON LAVEN. ChemInform Abstract: Preparation of Benzylphosphonates via a Palladium(0)-Catalyzed Cross-Coupling of H-Phosphonate Diesters with Benzyl Halides. Synthetic and Mechanistic Studies.[J]. ChemInform, 2010, 41 40. DOI:10.1002/chin.201040191.
[4] ANNA BRODZKA. The Synthesis and Evaluation of Diethyl Benzylphosphonates as Potential Antimicrobial Agents.[J]. Molecules, 2022. DOI:10.3390/molecules27206865.
diethyl benzylphosphonate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SYNOVA CHEMICALS | +91-9920741772 +91-9920741772 | Mumbai, India | 428 | 58 | Inquiry |
| Radison Labs Private Limited | +91-7337551966 +91-7337551966 | Hyderabad, India | 58 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 33024 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29858 | 58 | Inquiry |
| Accela ChemBio Inc. | +1-858-6993322 | United States | 19825 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SYNOVA CHEMICALS | 58 |
| Radison Labs Private Limited | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| CHEMSWORTH | 30 |
| ATK CHEMICAL COMPANY LIMITED | 60 |
| career henan chemical co | 58 |
| Accela ChemBio Inc. | 58 |
1080-32-6(diethyl benzylphosphonate)Related Search:
1of4
chevron_right




