Raltegravir
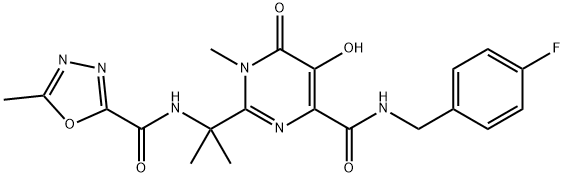
- CAS No.
- 518048-05-0
- Chemical Name:
- Raltegravir
- Synonyms
- CS-1889;Raltegravir;Reg column wei;Raltegravir(R&D);Raltegravir, >=99%;Raltegravir USP/EP/BP;Raltegravir(free base);Raltegravir (free base) API;MK-0518 518048-05-0 CAS NO.518048-05-0;Raltegravir-d4Q: What is Raltegravir-d4 Q: What is the CAS Number of Raltegravir-d4
- CBNumber:
- CB61456530
- Molecular Formula:
- C20H21FN6O5
- Molecular Weight:
- 444.42
- MOL File:
- 518048-05-0.mol
- Modify Date:
- 2024/7/25 20:04:51
| Melting point | 216 °C(Solv: isopropanol (67-63-0)) |
|---|---|
| Density | 1.46±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Very Slightly) |
| pka | 4.50±1.00(Predicted) |
| form | Solid |
| color | White to Light Beige |
| CAS DataBase Reference | 518048-05-0(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
Raltegravir Chemical Properties,Uses,Production
Description
Joining maraviroc as a unique approach to battling HIV-1, raltegravir, an inhibitor of HIV-1 integrase, represents the first in its class to be developed and launched as a combination treatment with other antiretroviral agents (NRTIs, NNRTIs, and PIs). HIV-1 integrase is essential for replication of the virus as a virally encoded enzyme that integrates the viral DNA into the genome of the host cell. Inhibition of HIV-1 integrase prevents the two-step process of endonucleolytic removal of the terminal dinucleotide from each 3′end of the viral DNA followed by the covalent integration of the viral DNA, at these modified 3′ends, into the host DNA, thereby representing a viable intervention in the viral life cycle. In vitro, raltegravir inhibited the strand transfer activity of HIV-1 integrase with an IC50 of 2–7nM with > 1,000-fold selectivity over other phosphoryltransferases. In addition, its in vitro IC95 for HIV-1 in 10% fetal bovine serum and 50% human serum was 19 and 33 nM, respectively. Raltegravir was well tolerated with no dose-related toxicities and a safety profile comparable to placebo. The most common clinical adverse events were diarrhea, nausea, vomiting, fatigue, headache, flushing, pruritus, and injection-site reactions.
Uses
Raltegravir (MK-0518, Isentress) is a potent integrase (IN) inhibitor for WT and S217Q PFV IN with IC50 of 90 nM and 40 nM, respectively.
Acquired resistance
Several characteristic mutations leading to typical amino acid exchanges have been characterized in cell culture studies and confirmed in clinical trial participants with virological failure while receiving raltegravir in combination with other antiretrovirals. Virological failure has generally been associated with mutations at one of three residues – Y143, Q148 or N155 – usually in combination with at least one other mutation.
Pharmaceutical Applications
Formulated as the potassium salt for oral administration.
Pharmacokinetics
Oral absorption: Not known/available
Cmax 400 mg twice daily: c. 2.17 mg/L
Plasma half-life: c. 9 h
Volume of distribution: Not known/available
Plasma protein binding: c. 83%
Absorption and distribution
It may be administered without regard to food. There are few data regarding its capacity to penetrate into genital secretions or breast milk. A study of 25 HIV-infected individuals receiving raltegravir as a component of combination antiretroviral therapy found that 24 had detectable levels and that 50% of these reached a level exceeding the 95% inhibitory concentration reported to inhibit HIV-1 strains fully susceptible to integrase inhibition.
Metabolism and excretion
It is not a substrate, and does not appear to inhibit or induce the cytochrome P450 enzyme complex. It is primarily metabolized through hepatic glucuronidation mediated by the UGT-1A1 enzyme. It is excreted in the feces (51%) and the urine (32%) as unaltered compound and its glucuronide. There are no recommended dose adjustments for weight, sex and race, or for hepatic or renal insufficiency. The pharmacokinetic handling in children has not been determined.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
Side effects
Its toxicity profile to date is remarkably benign. Clinical trial participants experienced similar types and frequencies of adverse events as those receiving placebo. The most frequently reported adverse events were nausea, diarrhea and headache and were mostly mild to moderate in intensity. Myopathy, rhabdomyolysis and elevations of creatinine phosphokinase have been noted in a few trial participants and it should be used cautiously in combination with drugs associated with muscle toxicity.
Raltegravir Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Laurus Labs Ltd | +91-4066594333 +91-4039804333 | Telangana, India | 50 | 58 | Inquiry |
| ShreeGen Pharma Ltd (part of Rumit Group of Companies) | +91-4023869399 +91-9664830616 | Telangana, India | 25 | 58 | Inquiry |
| Equilife Laboratories Pvt Ltd | +91-8007935656 +91-9822319778 | Maharashtra, India | 29 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Chiral Biosciences Limited | 09441920381 | Mallapur, India | 41 | 58 | Inquiry |
| Taj Mahal Vision Chemicals Private Limited | 07942557933 | Mumbai, India | 206 | 58 | Inquiry |
| Jigs Chemical Limited | +91 80 4290 2764 | Ahmedabad, India | 235 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
518048-05-0(Raltegravir)Related Search:
1of4
chevron_right




