Salcaprozate Sodium
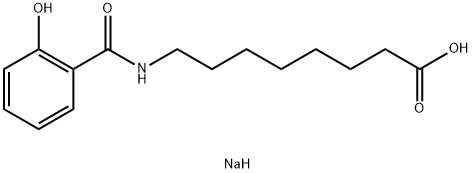
- CAS No.
- 203787-91-1
- Chemical Name:
- Salcaprozate Sodium
- Synonyms
- SNAC;sodium,8-[(2-hydroxybenzoyl)amino]octanoate;Salcaprozate Sodium (SNAC);sodium 8-(2-hydroxybenzamido)octanoate;Octanoic acid, 8-[(2-hydroxybenzoyl)amino]-, monosodium salt;E414;Salcaprozate sodium;SNAC reference standard;Chlorotoluron Impurity 3;8-(2-Hydroxybenzamido) acid sodium
- CBNumber:
- CB62739113
- Molecular Formula:
- C15H22NNaO4
- Molecular Weight:
- 303.33
- MOL File:
- 203787-91-1.mol
- Modify Date:
- 2024/11/15 19:19:13
| Melting point | 183 - 185°C |
|---|---|
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChI | InChI=1S/C15H21NO4.Na.H/c17-13-9-6-5-8-12(13)15(20)16-11-7-3-1-2-4-10-14(18)19;;/h5-6,8-9,17H,1-4,7,10-11H2,(H,16,20)(H,18,19);; |
| InChIKey | OBKUJEVXEMKQMJ-UHFFFAOYSA-N |
| SMILES | C(C1C=CC=CC=1O)(=O)NCCCCCCCC(=O)O.[NaH] |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
Salcaprozate Sodium Chemical Properties,Uses,Production
Description
Salcaprozate sodium (SNAC) is the sodium salt form of salcaprozate, an oral absorption promoter. Salcaprozate sodium may be used as a delivery agent to promote the oral absorption of certain macromolecules with poor bioavailability such as insulin and heparin.It is also used as an excipient in drug formulation as a chemical permeation enhancer (PE) to aid the oral absorption of macromolecules, peptides and proteins such as insulin (diabetes), heparin (heart attacks and angina) and cyanocobalamin (vitamin B12 deficiency and anaemia) which would otherwise have poor bioavailability. SNAC is considered to be safe for human consumption (GRAS) by the FDA.
Chemical Properties
It is a white to off-white crystalline powder, and its solubility in water is approximately 33 mg/mL.
Uses
Salcaprozate Sodium is a drug excipient or inactive ingredient in drug formulations.
Definition
Salcaprozate Sodium (SNAC) is a synthetic N-acetylated amino-acid derivative of salicylic acid. SNAC is a weak acid that display amphiphilicity and surface activity. It was discovered as part of a screen to identify carrier-based PEs that could “chaperone” poorly permeable payloads across the intestine. This compound is the most extensively tested carrier and the only PE approved in an oral formulation designed to improve oral BA, albeit with a small molecule, cyanocobalamin/SNAC. In other preclinical studies, it also improved intestinal permeation of peptides (salmon calcitonin (sCT) and insulin), along with poorly permeable small molecules (e.g., cromolyn). SNAC was tested in many formats: taste-masked liquids, tablets, and soft gelatin capsules. Similar to C10, SNAC can be blended with the active pharmaceutical ingredient (API) using conventional processes, which makes manufacturing uncoated oral tablet dosage forms economic and relatively easy to scale[1].
Side effects
Animal studies of Salcaprozate Sodium (SNAC) showed an associated mortality rate that was only evident at the 2000 mg/kg/day level, 20 per cent in males and 50 per cent in females; there was no clear cause of death. In the Wistar rat study, no mortality was observed at doses up to 1000 mg/kg/d. Clinicopathological parameters included slightly altered electrolyte levels and decreased globulin levels, and slightly higher liver and kidney weights, but no corresponding histopathological changes.Adverse effects of SNAC in Wistar rats were evident at levels up to 1000 mg/kg/d. The results of this study are summarised in the table below.
in vitro
SNAC (12.5-400 μg/mL; 24 h) has no toxicity to Caco-2 cells, and the survival percentage is above 90% when SNAC is 200 μg/mL.
SNAC (50 and 200 μg/mL) improves the apparent permeability coeffcient (Papp) of RA and SA-B by 2.14-fold and 3.68-fold compared with the Papp of SAs solution.
in vivo
SNAC improves the oral absorption of both R1 and SAs and enhances bioavailability in rats.
SNAC (2000 mg/kg/d; oral gavage for 13 weeks) related mortality is evident only at the 2000-mg/kg/d level, 20% among males and 50% among females; no clear cause of death is evident.
SNAC (100-1000 mg/kg/d; oral gavage for 13 weeks) induces no mortality in the Wistar rat study at doses up to 1000 mg/kg/d
Mode of action
The mode of action of Salcaprozate Sodium is controversial, and many scientists have explored its mechanism from various angles. Theory of oral semaglutide absorption, as advocated by Novo Nordisk. The optimum once-daily tablet consists of 14 mg of semaglutide co-formulated with 300 mg of SNAC. After digestion, the tablet erodes rapidly in the stomach, resulting in the release of a highly concentrated amount of SNAC that neutralizes the pH of gastric fluid in the immediate vicinity of the tablet to inactivate pepsin. SNAC is thought to induce semaglutide monomer production and increase gastric epithelial membrane fluidity, but without affecting tight junctions, thereby allowing transcellular passage of semaglutide into systemic circulation. Part of the role of SNAC seemed to be to convert semaglutide to a more permeable monomeric form and it seems to perform this better when formulated in a stomach-specific tablet[1].SNAC forms a conjugate base at the pH of the small intestinal lumen, so it can undergo complexation via hydrophobic ion pairing (HIP) with the conjugate acid of basic amino-acid side chains in macromolecules. However, HIP cannot fully account for Eligen-mediated hydrophobization of anionic payloads including heparin and cromolyn.
References
[1] Twarog C, et al. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C10). Pharmaceutics, 2019; 11: 78.
[2] SARINJ FATTAH . Salcaprozate sodium (SNAC) enhances permeability of octreotide across isolated rat and human intestinal epithelial mucosae in Ussing chambers[J]. European Journal of Pharmaceutical Sciences, 2020, 154: Article 105509. DOI:10.1016/j.ejps.2020.105509.
[3] M GARY I RILEY Ellen A P M Cristina Castelli. Subchronic oral toxicity of salcaprozate sodium (SNAC) in Sprague-Dawley and Wistar rats.[J]. International Journal of Toxicology, 2009, 28 4: 278-293. DOI:10.1177/1091581809337737.
Salcaprozate Sodium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Narmada Organics | +91-9601758907 +91-9409133000 | Gujarat, India | 19 | 58 | Inquiry |
| Apothecon Pharmaceuticals Pvt Ltd | +91-2662244212 +91-9099007601 | Gujarat, India | 28 | 58 | Inquiry |
| Blue Jet Healthcare Ltd | +91-02222071691 +91-9082861262 | Mumbai, India | 86 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| Hefei TianRui Pharmaceutical Chemical Co., Ltd. | +86-0551-68665055 +86-+86-18616906106 | China | 148 | 58 | Inquiry |
| XIAMEN SINOPEG BIOTECH CO., LTD. | +86-0592-7761068 +8617350879715 | China | 176 | 58 | Inquiry |
| Aceschem Inc. | +1-817863-6948 +1-(817)863-6948 | United States | 19632 | 58 | Inquiry |
| Biopole Pharmatech Co., Ltd. | +8615151475053 | China | 37 | 58 | Inquiry |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | China | 5972 | 58 | Inquiry |
Related articles
- Salcaprozate Sodium: Revolutionizing Oral Drug Delivery through Enhanced Gastrointestinal Absorption
- Salcaprozate sodium is a groundbreaking compound in the field of drug delivery, offering new possibilities for the oral admini....
- May 15,2024
- Salcaprozate Sodium: Uses, Side Effects and Related Research
- Salcaprozate Sodium (SNAC) is a synthetic N-acetylated amino acid derivative of salicylic acid widely used as a carrier for or....
- Mar 15,2024
- Salcaprozate Sodium Facilitates Oral Delivery of Low-Permeability Drugs
- Salcaprozate sodium enhances API absorption, potentially improving drug efficacy and bioavailability. Extensively studied in o....
- Jan 3,2024





