INACTIN
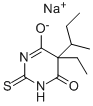
- CAS No.
- 947-08-0
- Chemical Name:
- INACTIN
- Synonyms
- inaktin;INACTIN;narkothion;brevinarcon;brevinarcone;venobarbital;Inactin? hydrate;Thiobutabarbital;thiobutabarbitonesodium;THIOBUTABARBITAL SODIUM
- CBNumber:
- CB6486701
- Molecular Formula:
- C10H15N2NaO2S
- Molecular Weight:
- 250.29
- MOL File:
- 947-08-0.mol
- Modify Date:
- 2024/3/19 15:37:50
| Melting point | 167-168 °C |
|---|---|
| solubility | H2O: storage of solutions for more than 8 hours at 4°C is not recommended.soluble |
| form | solid |
| color | light yellow |
| PH | pH:9.0~11.0 (50g/l, 25℃) |
| EPA Substance Registry System | Thiobutabarbital sodium (947-08-0) |
INACTIN Chemical Properties,Uses,Production
Uses
Inactin? hydrate has been used to anesthetize rats to study stress-induced hypersensitivity and mice to study Cisplatin-induced neuropathy. It has also been used to anesthetize rats to measure the glomerular filtration rate (GFR).
General Description
Crystals.
Air & Water Reactions
Slight hygroscopic. Water soluble.
Reactivity Profile
An amine, organosulfide. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data for INACTIN are not available. INACTIN is probably combustible.
INACTIN Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | China | 9708 | 58 | Inquiry |
| Wako Pure Chemical Industries, Ltd. | +81 6 6203 3741 | Japan | 6828 | 80 | Inquiry |
| SIGMA-RBI | 800 736 3690 (Orders) | Switzerland | 6913 | 91 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |
| 3B Scientific Corporation | 847.281.9822 | United States | 6744 | 47 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| Lanospharma Laboratories Co.,Ltd | 13440048448 | China | 6343 | 56 | Inquiry |
| Riedel-de Haen AG | 800 558-9160 | United States | 6825 | 87 | Inquiry |
| Shanghai Xiyuan Biotechnology Co., Ltd | 021-58116080 | CHINA | 6936 | 58 | Inquiry |





