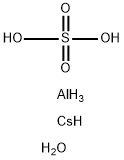
ALUMINUM CESIUM SULFATE
- CAS No.
- 7784-17-0
- Chemical Name:
- ALUMINUM CESIUM SULFATE
- Synonyms
- CESIUM ALUM;CESIUM ALUMINUM SULFATE;ALUMINUM CESIUM SULFATE;Aluminum cesium sulfate 1dihydrate;aluminumcesiumsulfatedodecahydrate;ALUMINUM CESIUM SULFATE DODECAHYDRATE, 9 9.99+%
- CBNumber:
- CB6715801
- Molecular Formula:
- AlCsH8O5S
- Molecular Weight:
- 280.01
- MOL File:
- 7784-17-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:54
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H335-H315 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
ALUMINUM CESIUM SULFATE Chemical Properties,Uses,Production
Chemical Properties
colorless, cub crystal(s); used in mineral waters [HAW93] [CRC10]
Production Methods
The alkali metals and Al are present in pollucite in about the right proportions for the formation of alum. Hence, Clusius and Stern can follow the procedure. The mineral is decomposed with hydrochloric acid, and Cs is precipitated as a low-solubility alum by treatment with sulfuric acid. Thus, 0.5 kg of very finely pulverized (0.01 mm.) pollucite in one liter of 18% hydrochloric acid is evaporated to dryness on a water bath. This procedure is repeated three times, and each time the dry residue is extracted with one liter of HaO + 100 ml. of concentrated hydrochloric acid. This is followed by suction filtration on a filter cloth. The filtered extracts are combined and concentrated to one liter, and the silicic acid, which separates almost completely, is decanted. The alum is then gradually precipitated with 200 ml. of concentrated H3SO4. After cooling, about 545 g of crude yellowish alum is obtained. The mother liquor is practically free of Cs. The crystallization is repeated several times, dissolving 250 g of the alum in 2.5 liters of boiling water in a four-liter Erlenmeyer flask. On slow cooling (constant agitation, 10 hours), the alum again separates. The material obtained after six crystallizations shows no traces of other alkali metals. One way to ensure pure alum is to check the purity of the mother liquor from which it is precipitated.
Purification Methods
Recrystallise it from hot water (3mL/g).
ALUMINUM CESIUM SULFATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21628 | 55 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +86-18621343501; +undefined18621343501 | China | 33338 | 58 | Inquiry |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | China | 40066 | 58 | Inquiry |
| DAYANG CHEM (HANGZHOU) CO.,LTD | +8617705817739 | China | 53881 | 58 | Inquiry |
| Shanghai Maclean Biochemical Technology Co., LTD | 021-50706066 15221275939 | China | 29784 | 58 | Inquiry |
| Baoji Didu Pharmaceutical and Chemical Co., Ltd | 029-81148929 13186179623 | China | 9946 | 58 | Inquiry |
| Absin Bioscience Inc. | 021-38015121-8802 | CHINA | 6208 | 58 | Inquiry |
| Zhengzhou Alpha Chemical Co. LTD | 0371-0371-53732842 13383863444 | China | 9999 | 58 | Inquiry |
| HUNAN CHEMFISH SCIENTIFIC CO.,LTD | 0731-+86-0731-85567275 18932438858 | China | 6710 | 58 | Inquiry |
| Shanghai Haohong Pharmaceutical Co., Ltd. | 400-8210725 4008210725 | China | 55017 | 58 | Inquiry |





