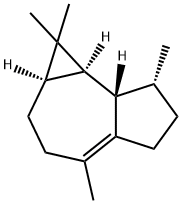
(+)-Ledene
- CAS No.
- 21747-46-6
- Chemical Name:
- (+)-Ledene
- Synonyms
- (+)-Ledene;Viridflorene;Varidiflorene;LEDENE, (+)-(RG);Einecs 244-565-3;Guaiazulene Impurity 4;95.0% (sum of enantiomers, GC);(+)-LEDENE 95+% MIXTURE OF ISOMERS;(+)-Ledene >=95.0% (sum of enantiomers, GC);(1S,2R,3R,11R)-3,3,7,11-TETRAMETHYL-TRICYCLO[6.3.0.0(2.4)]UNDEC-7-ENE
- CBNumber:
- CB7153554
- Molecular Formula:
- C15H24
- Molecular Weight:
- 204.35
- MOL File:
- 21747-46-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 269°C |
|---|---|
| Boiling point | 268-270 °C (lit.) |
| Density | 0.927 g/mL at 20 °C (lit.) |
| refractive index |
n |
| storage temp. | Refrigerator |
| solubility | Chloroform (Sparingly), Ethanol (Slightly) |
| form | Oil |
| color | Colourless |
| optical activity | [α]20/D +68±2°, c = 10% in ethanol |
| BRN | 2554786 |
| LogP | 6.447 (est) |
(+)-Ledene Chemical Properties,Uses,Production
Description
(+)-Ledene, also called leden or viridiflorene, is an organic compound that belongs to the group of 5, 10-cycloaromadendrane sesquiterpenoids. These compounds are derived from the aromadendrane skeleton through a cyclization reaction between carbon atoms 5 and 10. (+)-Ledene is mainly found in saliva and is considered to have low solubility in water and a relatively neutral nature. In cellular environments, (+)-ledene is predominantly present in the cell membrane (predicted based on logP) and cytoplasm.
Uses
(+)-Ledene is an essential oil that is synthesized by viridiflorene synthase. It has been studied as an antioxidant, antimicrobial, antibiofilm and to treat various cancers.
Definition
ChEBI: Viridiflorene is a carbotricyclic sesquiterpene obtained from several natural sources, including Australian Tea Tree oil (Melaleuca alternifolia.) It is a sesquiterpene and a carbotricyclic compound.
General Description
(+)-Ledene is a naturally occurring sesquiterpene that can be prepared from (+)-aromadendrene via isomerization.
References
[1] DUC N. TRAN Prof.??Dr. N C. Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (?)-Palustrol, (+)-Spathulenol, and Psiguadial A, C, and D via the Platform Terpene (+)-Bicyclogermacrene[J]. Chemistry - A European Journal, 2014. DOI:10.1002/chem.201403082.
[2] S. L. GWALTNEY K. J S S T Sakata. Bridged to Fused Ring Interchange. Methodology for the Construction of Fused Cycloheptanes and Cyclooctanes. Total Syntheses of Ledol, Ledene, and Compressanolide[J]. The Journal of Organic Chemistry, 1996. DOI:10.1021/jo961005a.
(+)-Ledene Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30231 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8772 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +86-18621343501; +undefined18621343501 | China | 33338 | 58 | Inquiry |
| Aladdin Scientific | United States | 52924 | 58 | Inquiry | |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | China | 40066 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131957 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6831 | 75 | Inquiry |
| Shanghai YuanYe Biotechnology Co., Ltd. | 021-61312847; 18021002903 | China | 71829 | 60 | Inquiry |





