Tepotinib
- CAS No.
- 1100598-32-0
- Chemical Name:
- Tepotinib
- Synonyms
- Tepotinib;EMD-1214063;Veledimex;CS-977;MSC2156119;EMD1214063,Tepotinib;epotinib(EMD 1214063);Tepotinib (EMD 1214063);EMD 1214063; EMD1214063;EMD-1214063|||MSC2156119
- CBNumber:
- CB72550731
- Molecular Formula:
- C29H28N6O2
- Molecular Weight:
- 492.57
- MOL File:
- 1100598-32-0.mol
- Modify Date:
- 2024/7/2 8:55:16
| Boiling point | 626.5±65.0 °C(Predicted) |
|---|---|
| Density | 1.25 |
| storage temp. | Store at -20°C |
| form | Solid |
| pka | 8.93±0.10(Predicted) |
| Water Solubility | ≥ 4.93 mg/mL in DMSO, <2.52 mg/mL in EtOH, <2.56 mg/mL in Water |
| InChIKey | AHYMHWXQRWRBKT-UHFFFAOYSA-N |
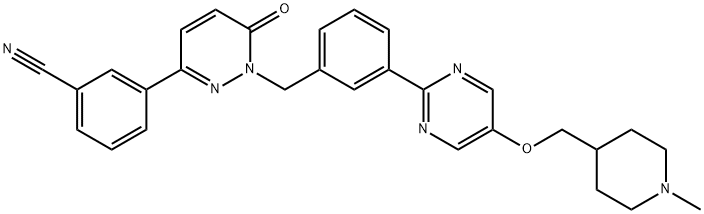
| SMILES | C(#N)C1=CC=CC(C2=NN(CC3=CC=CC(C4=NC=C(OCC5CCN(C)CC5)C=N4)=C3)C(=O)C=C2)=C1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
Tepotinib Chemical Properties,Uses,Production
Description
Tepotinib is a highly selective inhibitor against MET. In xenograft models, acquired resistance to EGFR TKIs via secondary EGFR T790 M mutations can be overcome with tepotinib treatment.
Uses
EMD 1214063 is a novel ATP-comptetitive inhibitor of the MET hepatocyte growth factor receptor and a novel kinase inhibitor and a therapeutic agent for neuroblastoma. Potent c-MET inhibitor.
brand name
Tepmetko
General Description
Class: receptor tyrosine kinase; Treatment: NSCLC with METex14; Other name: EMD-1214063; Oral bioavailability = 72%; Elimination half-life = 32 h; Protein binding = 94%
Mechanism of action
Tepotinib is a Kinase Inhibitor. The mechanism of action of tepotinib is as a Mesenchymal Epithelial Transition Inhibitor, and P-Glycoprotein Inhibitor.
Pharmacology
Tepotinib is a highly-selective inhibitor of MET kinase activity, with an average IC50 of approximately 1.7 nmol/L. It has a moderate duration of action necessitating once-daily administration. Tepotinib has been associated with the development of interstitial lung disease (ILD)/pneumonitis, which can sometimes be fatal. Patients should be monitored closely for signs of new or worsening respiratory symptoms (e.g. dyspnea, cough), and treatment with tepotinib should be immediately withheld if ILD/pneumonitis is suspected. If no other potential causes of ILD/pneumonitis are identified, therapy with tepotinib should be suspended indefinitely.
Clinical Use
Tepotinib is currently being evaluated in combination with EGFR TKI gefitinib and also a separate trial in NSCLC patients with MET exon 14 skipping mutation and MET amplification.
Side effects
- anxiety
- chest pain or tightness
- difficult or labored breathing
- dizziness or lightheadedness
- fast heartbeat
- fever or chills
- general feeling of discomfort or illness
- muscle or bone pain
Synthesis
The synthesis of Tepotinib is as follows:
Acetonitrile (700 ml) was added to the reaction vessel, and the compound represented by formula 7a (37.63 g, 0.1 mol), the compound represented by formula 8 (23.66 g, 0.12 mol), and potassium carbonate (34.55 g, 0.25 mol) were added under stirring.and tetrabutylammonium bromide (1.93g, 0.006mol), the reaction system was stirred and heated to reflux, and the reaction was stirred at reflux for 12h. After the reaction was completed, the temperature was cooled to room temperature, the reaction solution was concentrated under reduced pressure to dryness, and ethanol (360ml) was added. ), stir to dissolve, slowly add 1.5 mol/l hydrochloric acid aqueous solution dropwise, adjust the pH value to 1-2, cool down to 5-7 °C, stir and crystallize for 6 h, filter, and vacuum dry the solid at 45 °C for 6 h to obtain formula 1. The compound (44.42 g) was shown in a yield of 81.2% and a purity of 99.7% by HPLC.
target
Primary target: MET
Drug interactions
Tepotinib is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) who have mesenchymal-epithelial transition (_MET_) exon 14 skipping alterations.
Metabolism
Tepotinib is metabolized primarily by CYP3A4 and CYP2C8, with some apparent contribution by unspecified UGT enzymes. The metabolite M506 is the major circulating metabolite, comprising approximately 40.4% of observed drug material in plasma, while the M668 glucuronide metabolite has been observed in plasma at much lower quantities (~4% of an orally administered dose). A total of 10 phase I and phase II metabolites have been detected following tepotinib administration, most of which are excreted in the feces.
Tepotinib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 25247 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10522 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Shanghai Innopharm Industrial Co., Ltd. | +86-21-59960785 +86-17701845089 | China | 338 | 58 | Inquiry |
Related articles
- How is Tepotinib Synthesised?
- Tepotinib is synthesised through a two-step chemical reaction using acetylbenzonitrile as the raw material.
- Jan 8,2024
1100598-32-0(Tepotinib)Related Search:
1of2
chevron_right




