Zinc acetate
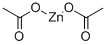
- CAS No.
- 557-34-6
- Chemical Name:
- Zinc acetate
- Synonyms
- Zn(OAc)2;Zinc diacetate;Galzin;NA-9153;ai3-04465;siltexcl4;ZINC ACETATE;Diacetoxyzinc;zincdiacetate;ZincAcetateBp
- CBNumber:
- CB8108591
- Molecular Formula:
- C4H6O4Zn
- Molecular Weight:
- 183.48
- MOL File:
- 557-34-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/14 20:44:35
| Melting point | 83-86 °C |
|---|---|
| Boiling point | 908°C |
| Density | 1.84 g/mL at 25 °C (lit.) |
| vapor pressure | 0.001Pa at 25℃ |
| Flash point | 12 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Methanol (Slightly), Water (Slightly) |
| pka | 4.756[at 20 ℃] |
| form | Powder |
| Specific Gravity | 1.84 |
| color | Yellow to brown to gray-green |
| Water Solubility | Soluble in water, alcohol, dilute mineral acids and alkalies. |
| Sensitive | Hygroscopic |
| Merck | 14,10128 |
| InChIKey | DJWUNCQRNNEAKC-UHFFFAOYSA-L |
| LogP | -1.28 |
| CAS DataBase Reference | 557-34-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Zinc acetate(557-34-6) |
| EPA Substance Registry System | Zinc acetate (557-34-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H318-H411 | |||||||||
| Precautionary statements | P264-P270-P273-P280-P301+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N,Xi,F | |||||||||
| Risk Statements | 36-50/53-22-11 | |||||||||
| Safety Statements | 26-60-61-39-16-7 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AK1500000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| HS Code | 29152990 | |||||||||
| NFPA 704 |
|
Zinc acetate price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 383317 | Zinc acetate 99.99% trace metals basis | 557-34-6 | 25G | ₹9820 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 383317 | Zinc acetate 99.99% trace metals basis | 557-34-6 | 100G | ₹29822.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 383317 | Zinc acetate 99.99% trace metals basis | 557-34-6 | 500G | ₹50704.3 | 2022-06-14 | Buy |
| ALFA India | ALF-035792-18 | Zinc acetate, anhydrous, 99.98% (metals basis) | 557-34-6 | 50g | ₹16134 | 2022-05-26 | Buy |
| ALFA India | ALF-035792-09 | Zinc acetate, anhydrous, 99.98% (metals basis) | 557-34-6 | 10g | ₹4924 | 2022-05-26 | Buy |
Zinc acetate Chemical Properties,Uses,Production
Description
Zinc acetate (chemical formula: Zn(O2CCH3)2) is a kind of salt commonly existing in the dihydrate form. It appears as colorless solid. It is produced through the reaction between zinc oxide with acetate acid. Given that zinc is an essential element for growth and development of human body, zinc acetate can be used as a dietary supplement for the treatment of zinc deficiency. It plays an important role for the synthesis of cholesterol, protein, and fats. It can also be used as an astringent, styptic and emetic. In industry, it has various kinds of applications including wood preservation, manufacturing of other zinc salts as well as ethylene acetate, being used as a dye mordant, and analytical reagent.
Chemical Properties
Zinc acetate occurs as white crystalline, lustrous plates with a faint acetic odor and an astringent taste.
Physical properties
The acetate group is capable of binding to metal ions in a variety of ways through its two oxygen atoms and several connectivities are observed for the various hydrates of zinc acetate. Anhydrous zinc acetate adopts a polymeric structure consisting of zinc coordinated to four oxygen atoms in a tetrahedral environment, each tetrahedron being connected to neighbors by the acetate groups . The acetate ligands are not bidentate. In contrast, most metal diacetates feature metals in octahedral coordination with bidentate acetate groups. In zinc acetate dihydrate the zinc is octahedral, wherein both acetate groups are bidentate.
2 - 5 - Heating pad
Heating Zn(CH3CO2)2 in a vacuum results in loss of acetic anhydride, leaving a residue of ""basic zinc acetate,"" with the formula Zn4O(CH3CO2)6. This cluster compound has the tetrahedral structure shown below. This species closely resembles the corresponding beryllium compound, although it is slightly expanded with Zn-O distances ~1.97 vs ~1.63 Å for Be4O(OAc)6.
Preparation
Zinc acetate is prepared by the reaction of acetic acid with zinc oxide followed by crystallization (crystals of dihydrate obtained): ZnO + 2CH3COOH → (CH3COO)2Zn + H2O.
Production Methods
Zinc acetate is synthesized by reacting zinc oxide with glacial acetic acid, with subsequent crystallization, separation by centrifugation, and drying and milling of the crystals. No organic solvents are used during the synthesis.
Definition
ChEBI: Zinc acetate is an acetate salt in which the cationic component is zinc(2+). It has a role as an astringent. It is a zinc molecular entity and an acetate salt.
Application
Dietary and medicinal applications
Zinc acetate is used as a dietary supplement and in lozenges used to treat the common cold. Zinc acetate alone is thought to be a more effective treatment than zinc gluconate. Zinc acetate can also be used to treat zinc deficiencies. As an oral daily supplement it is used to inhibit the body's absorption of copper as part of the treatment for Wilson's disease. Zinc acetate is also sold as an astringent in the form of an ointment, a topical lotion; or combined with an antibiotic such as erythromycin for the topical treatment of acne. Furthermore Zinc acetate is commonly sold as a topical anti-itch ointment.
Industrial applications
Industrial applications include wood preserving, manufacturing other zinc salts, polymers, manufacture of ethylene acetate, as a dye mordant, and analytical reagent. Zinc acetate is a precursor via a sol-gel route to the transparent semi conductor zinc oxide. Zinc acetate is also used for manufacturing glazers for painting on porcelain; as a reagent in testing for albumin, tannin, phosphate; as cross-linking agents for polymers; in tobacco smoke filters; and as a topical fungicide.
Pharmaceutical Applications
Zinc acetate has been used as an excipient in a variety of
pharmaceutical formulations including topical gels, lotions, and
solutions, and subcutaneous injections. It has also been investigated
for use in an oral controlled-release formulation for water-soluble
drugs in combination with sodium alginate and xanthan gum.
Therapeutically, zinc acetate has been used in oral capsules for
the treatment of Wilson’s disease. Zinc acetate has also been
demonstrated to be effective as a spermicide in vaginal contraceptives.
Safety
Zinc acetate is used in topical pharmaceutical formulations and
subcutaneous injections, where it is generally regarded as relatively
nontoxic and nonirritant when used as an excipient. However, zinc
acetate is poisonous by intravenous and intraperitoneal routes; it is
also moderately toxic following oral consumption.
Zinc acetate:
LD50 (rat, oral): 2.510 g/kg
LD50 (mouse, IP): 0.057 g/kg
storage
Zinc acetate loses water of hydration above 101℃. Zinc acetate should be stored in an airtight container in a cool, dry, place.
Incompatibilities
Zinc acetate is incompatible with oxidizing agents, zinc salts, alkalis and their carbonates, oxalates, phosphates, and sulfides.
Regulatory Status
Included in the FDA Inactive Ingredients Database (SC injections; topical lotions and solutions). Included in medicines licensed in the UK.
References
https://en.wikipedia.org/wiki/Zinc_acetate
https://pubchem.ncbi.nlm.nih.gov/compound/Zinc_acetate_dihydrate#section=GHS-Classification
Zinc acetate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| SAGAR LIFE SCIENCES PVT LTD | +91-8770359041 +91-8770359041 | Gujarat, India | 79 | 58 | Inquiry |
| Indiana ChemPort | +91-91-2652638667 +91-7574886711 | Gujarat, India | 85 | 58 | Inquiry |
| S.V ENTERPRISES | +919322701159 | Mumbai, India | 150 | 58 | Inquiry |
| ALLIPO CHEMICALS | +91-8238998187 +91-8238998187 | Gujarat, India | 32 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| AVA CHEMICALS PVT LTD | +91-8879390046 +91-8879390046 | Maharashtra, India | 74 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| Salicylates and Chemicals Pvt. Ltd. | +91-8879630551 +91-8898005838 | Maharashtra, India | 61 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| SAGAR LIFE SCIENCES PVT LTD | 58 |
| Indiana ChemPort | 58 |
| S.V ENTERPRISES | 58 |
| ALLIPO CHEMICALS | 58 |
| Evans Fine Chem | 58 |
| AVA CHEMICALS PVT LTD | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| Dhara Industries | 58 |
| Salicylates and Chemicals Pvt. Ltd. | 58 |
557-34-6(Zinc acetate)Related Search:
1of4
chevron_right




