BARIUM CHLORATE MONOHYDRATE
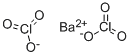
- CAS No.
- 13477-00-4
- Chemical Name:
- BARIUM CHLORATE MONOHYDRATE
- Synonyms
- bachlorate;cloratobarico;BARIUM CHLORATE;bariumchlorate,wet;BARIUM CHLORATE 99%;BARIUM CHLORATE MONO;barium(2+) dichlorate;Chloricacidbariumsalt;Chloricacid,bariumsalt;bariuM chlorate hydrate
- CBNumber:
- CB8143644
- Molecular Formula:
- BaCl2H2O7
- Molecular Weight:
- 322.24
- MOL File:
- 13477-00-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | 414 °C(lit.) |
|---|---|
| Density | 3.18 g/mL at 25 °C(lit.) |
| solubility | slightly soluble in ethanol, acetone |
| color | Colorless prisms or white powder |
| Water Solubility | 27.5g/100g solution (25°C); 67g/100g solution (100°C) [CIC73] |
| CAS DataBase Reference | 13477-00-4(CAS DataBase Reference) |
| EPA Substance Registry System | Chloric acid, barium salt (2:1) (13477-00-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS09,GHS03 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H271-H302-H332-H411 | |||||||||
| Precautionary statements | P210-P220-P221-P280-P283-P306+P360-P371+P380+P375-P370+P378-P501-P264-P270-P301+P312-P330-P501-P261-P271-P304+P340-P312 | |||||||||
| Hazard Codes | O,Xn,N | |||||||||
| Risk Statements | 9-20/22-51/53 | |||||||||
| Safety Statements | 13-27-61 | |||||||||
| RIDADR | UN 1445 5.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
BARIUM CHLORATE MONOHYDRATE Chemical Properties,Uses,Production
Description
Barium chlorate has the molecular formula of
Ba(ClO3)2 and a molecular weight of 304.229 g/mol. Its
density is 3.18 g/cm3 and its melting point is 413.9°C.
It is a white crystalline solid, a skin irritant and if
consumed can cause nausea, vomiting, and diarrhea.
Its CAS number is 13477-00-4. It is used in fireworks to
produce a green color. It also forms a monohydrate,
Ba(ClO3)2·H2O whose CAS number is 10294-38-9.
Shock-sensitive compounds are formed with organic
compounds, reducing agents, ammonia-containing
agents, and metal powders. The substance decomposes
violently on heating, producing oxygen, toxic fumes,
and causing fire and explosive hazards. This salt is
a strong oxidant and reacts with most combustible and
reducing materials.
Chemical Properties
Barium chlorate is a combustible, colorless to white crystalline solid or powder.
Uses
Pyrotechnics, explosives, textile mordant, man- ufacture of other chlorates.
Preparation
Barium chlorate can be produced through a double
replacement reaction of barium chloride and sodium
chlorate:
BaCl2+ 2NaClO3→Ba(ClO3)2+ 2NaCl
It can also be produced through a more complicated
process involving barium carbonate and ammonium chlorate, both of which are produced in situ. There are
four separate reactions used to produce this salt:
BaCl2+ Na2CO3 →BaCO3+ 2NaCl or BaCl2+2NaHCO3 →BaCO3+ 2NaCl+ H2O+ CO2(1)
In a separate step, ammonium chlorate is produced
by reaction of tartaric acid, C4H6O6, to produce ammonium
bitartrate. This is then reacted with potassium
chlorate which produces potassium bitartrate and
ammonium chlorate:
C4H6O6+NH4OH→NH4C4H5O6+H2O (2)
NH4C4H5O6+ KClO3→KC4H5O6+NH4ClO3 (3)
The produced barium carbonate is then reacted with
ammonium chlorate:
2NH4ClO3+ BaCO3+ heat→Ba(ClO3)2+ 2NH3+H2O+ CO2 (4)
The final product is obtained without having to handle
the chlorate solutions or product before the final product
is obtained.
It can also be produced via the Liebig process similar
to that of the strontium homologue which consists of
passing chlorine gas through a solid such as Ba(OH)2:
6Ba(OH)2+ 6Cl2→5BaCl2+ Ba(CIO3)2+ 6H2O
However, separating the two salts remains problematic
since both are soluble in water. It is for
this reason that the Liebig method is not used for the
commercial production of barium chlorate.
Hazard
A poison. Strong oxidizer, fire risk in con- tact with organic materials.
Safety Profile
A poison. For fire and explosion hazards, see CHLORATES. Incompatible with Al, As, C, charcoal, Cu, MnO2, metal sulfides, S4N4, organic matter, P, S. See also BARIUM COMPOUNDS (soluble).
Potential Exposure
It is used in fireworks and explosives manufacture; in textile dyeing and in the manufacture of other perchlorates.
Shipping
UN1445 Barium chlorate, Hazard Class: 5.1; Labels: 5.1—Oxidizer, 6.1—Poisonous materials.
Purification Methods
It crystallises from H2O (1mL/g) between 100o and 0o, and loses 1H2O at 120o. [Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 314 1963.]
Incompatibilities
A strong oxidizer. When heated above 250° C, it begins to give off oxygen and will increase risk of fire. Barium chlorate is a reactive chemical and is an explosion hazard. Violent reaction may occur with reducing materials; strong acids; powdered metals. Contact with combustible materials will increase activity in fire.
Waste Disposal
Use large volumes of reducing agent (bisulfite or ferrous salt) solutions. Neutralize and flush to sewer with large volumes of water.
BARIUM CHLORATE MONOHYDRATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 15093356674; | China | 29826 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9020 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |
| Shenzhen Longteng Chemical Co., Ltd | 13049850850 | China | 1 | 58 | Inquiry |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-63373950 15353716720 | China | 10011 | 58 | Inquiry |
| Portail Substances Chimiques | 10 20 0000 | France | 6027 | 58 | Inquiry |
| Shandong Xiya Chemical Co., Ltd. | 4009903999 13395398332 | China | 20810 | 60 | Inquiry |
| Riedel-de Haen AG | 800 558-9160 | United States | 6825 | 87 | Inquiry |
| Spectrum Chemicals & Laboratory Products | 310 516 8000 | United States | 6706 | 77 | Inquiry |
13477-00-4(BARIUM CHLORATE MONOHYDRATE)Related Search:
1of4
chevron_right




