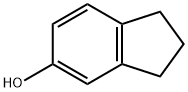
5-INDANOL
- CAS No.
- 1470-94-6
- Chemical Name:
- 5-INDANOL
- Synonyms
- 1H-Inden-5-ol, 2,3-dihydro-;2,3-Dihydro-1H-inden-5-ol;INDAN-5-OL;5-HYDROXYINDAN;5-HYDROXYINDANE;5-INDANOL;Indanol-5;5-INDANOL 99%;5-Indanol,99%;Indan-5-ol 98%
- CBNumber:
- CB8210012
- Molecular Formula:
- C9H10O
- Molecular Weight:
- 134.18
- MOL File:
- 1470-94-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/12/22 17:53:22
| Melting point | 51-53 °C (lit.) |
|---|---|
| Boiling point | 255 °C (lit.) |
| Density | 0.8540 (rough estimate) |
| refractive index | 1.5630 (estimate) |
| Flash point | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 6.3g/l |
| form | powder to crystal |
| pka | 10.37±0.20(Predicted) |
| color | slightly brown |
| BRN | 1936314 |
| CAS DataBase Reference | 1470-94-6(CAS DataBase Reference) |
| EPA Substance Registry System | 5-Indanol (1470-94-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H311 | |||||||||
| Precautionary statements | P280-P312 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 21-20/21/22 | |||||||||
| Safety Statements | 36/37-36 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NK7525000 | |||||||||
| Hazard Note | Irritant | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29062900 | |||||||||
| NFPA 704 |
|
5-INDANOL price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | I2215 | 5-Indanol 99% | 1470-94-6 | 25G | ₹5282.6 | 2022-06-14 | Buy |
| TCI Chemicals (India) | H0251 | 5-Hydroxyindan min. 99.0 % | 1470-94-6 | 25G | ₹6000 | 2022-05-26 | Buy |
| TCI Chemicals (India) | H0251 | 5-Hydroxyindan min. 99.0 % | 1470-94-6 | 100G | ₹15800 | 2022-05-26 | Buy |
5-INDANOL Chemical Properties,Uses,Production
Description
5-Indanol, mp 54–55 ℃, bp 255 ℃, is produced from indan [496-11-7] by a sulfonation–alkali-fusion process. A synthesis for 1,1,3,3-tetraalkylindanols was developed by Bayer, which involves the reaction of isoolefins with secondary alkenylphenols (or compounds generating them under the reaction conditions) at 100–250 ℃ and in the presence of acidic catalysts. An example is the reaction of isobutene with bisphenol A in the presence of acid-activated clays which yields 1,1,3,3-tetramethyl-5-indanol [53718-26-6], mp 115 ℃, bp (2.66 kPa) 156 ℃, in a yield of 80% of the theoretical, and 4-tert-butylphenol as a coproduct.
Chemical Properties
light brown to grey-brown crystalline powder or
Definition
ChEBI: A member of the class of phenols that is indan which has been hydroxylated at position 5.
Purification Methods
Crystallise 5-hydroxyindane from pet ether (m 56o) or pentane (m 59-60o). It has UV with max at 283.5nm (cyclohexane). The 3,5-dinitrobenzoyl derivative has m 156o. [Beilstein 6 III 2428, 6 IV 3829.]
5-INDANOL Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SANTO RIGHELLO PVT ltd | +91 9885571757 | Telangana, India | 41 | 58 | Inquiry |
| Oceanic Pharmachem Pvt. Ltd. | 91-22-42128600 | Maharashtra, India | 2006 | 58 | Inquiry |
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6211 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| A. B. Enterprises | +91-8048077585 | Mumbai, India | 1396 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20296 | 58 | Inquiry |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | China | 2141 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | China | 5738 | 58 | Inquiry |
1470-94-6(5-INDANOL)Related Search:
1of4
chevron_right




