ChemicalBook > Product Catalog >Analytical Chemistry >Standard >Pharmaceutical Impurity Reference Standards >Ondansetron HCl IMpurity-G
Ondansetron HCl IMpurity-G
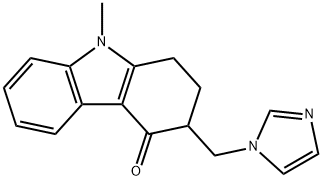
- CAS No.
- 99614-03-6
- Chemical Name:
- Ondansetron HCl IMpurity-G
- Synonyms
- C-Demethylondansetron;Ondasatrone Impurity G;C-Desmethyl Ondansetron;Ondansetron HCl IMpurity-G;(3RS)-3-[(1H-Imidazol-1-yl)methyl]-;Ondansetron Impurity 7(Ondansetron EP Impurity G);Ondansetron Hydrochloride Dihydrate EP Impurity G;Ondansetron EP Impurity G (C-Desmethyl Ondansetron);3-(imidazol-1-ylmethyl)-9-methyl-2,3-dihydro-1H-carbazol-4-one;1,2,3,9-Tetrahydro-3-(1H-imidazol-1-ylmethyl)-9-methyl-4H-carbazol-4-one
- CBNumber:
- CB82670263
- Molecular Formula:
- C17H17N3O
- Molecular Weight:
- 279.34
- MOL File:
- 99614-03-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/7/12 17:11:13
| Melting point | 185 - 188°C |
|---|---|
| Boiling point | 549.1±30.0 °C(Predicted) |
| Density | 1.29±0.1 g/cm3(Predicted) |
| storage temp. | Room Temperature |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), DMSO (Slightly) |
| pka | 6.75±0.12(Predicted) |
| form | Solid |
| color | White to Off-White |
Ondansetron HCl IMpurity-G Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 55)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| Molsyns Research | +91-9586858886 +91-9586858886 | Gujarat, India | 418 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| sgtlifesciences pvt ltd | +8617013299288 | China | 12382 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | China | 11927 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131980 | 58 | Inquiry |
| Guangzhou Dreampharm Biotechnology Co., Ltd. | 020-36607679 17825480238 | China | 9875 | 12 | Inquiry |
99614-03-6(Ondansetron HCl IMpurity-G)Related Search:
Ondansetron HCl IMpurity-G
1,2,3,9-Tetrahydro-3-(1H-imidazol-1-ylmethyl)-9-methyl-4H-carbazol-4-one
(Ondansetron Impurity G)
1,2,3,9-Tetrahydro-3-(1H-imidazol-1-ylmethyl)-9-methyl-4H-carbazol-4-one
Ondansetron Impurity 7(Ondansetron EP Impurity G)
3-(imidazol-1-ylmethyl)-9-methyl-2,3-dihydro-1H-carbazol-4-one
Ondansetron Hydrochloride Dihydrate EP Impurity G
(3RS)-3-[(1H-Imidazol-1-yl)methyl]-
4H-Carbazol-4-one, 1,2,3,9-tetrahydro-3-(1H-imidazol-1-ylmethyl)-9-methyl-
Ondansetron EP Impurity G (C-Desmethyl Ondansetron)
C-Desmethyl Ondansetron
(3RS)-3-[(1h-imidazol-1-yl)methyl]-9-methyl-1,2,3,9-tetrahydro-4h-carbazol-4-one(c-desmethylondansetron),
3-((1H-Imidazol-1-yl)methyl)-9-methyl-2,3-dihydro-1H-carbazol-4(9H)-one (Ondansetron Impurity)
Ondansetron EP Impurity G
3-((1H-imidazol-1-yl)methyl)-9-methyl-1,2,3,9-tetrahydro-4H- carbazol-4-one
C-Demethylondansetron
Ondasatrone Impurity G
99614-03-6





