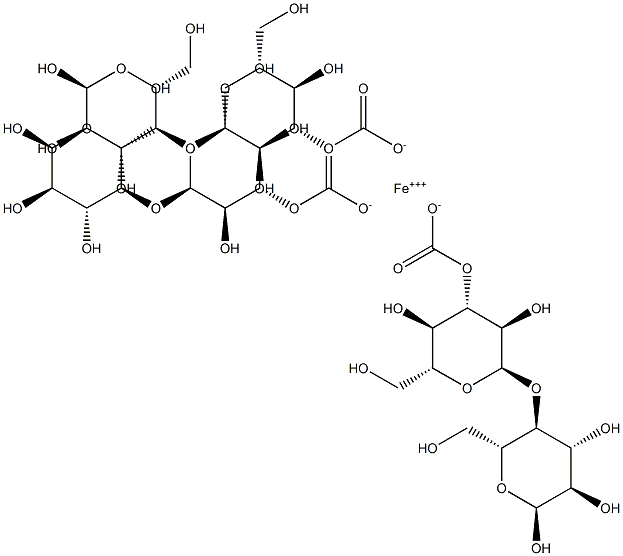
Ferric carboxymaltose
- CAS No.
- 9007-72-1
- Chemical Name:
- Ferric carboxymaltose
- Synonyms
- Ferinject;Injectafer;Ferric carboxymahose;Ferric Carboxymaltos;Injectafer USP/EP/BP;Iron carboxymaltose;Iron Dextri-Maltose;Ferric carboxymaltose;Ferric Carboxymaltose Injectafer
- CBNumber:
- CB82717081
- Molecular Formula:
- C39H63FeO39
- Molecular Weight:
- 1211.73912
- MOL File:
- 9007-72-1.mol
- Modify Date:
- 2024/4/9 22:15:29
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H315-H335-H373-H361 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362-P260-P314-P501-P201-P202-P281-P308+P313-P405-P501 |
Ferric carboxymaltose Chemical Properties,Uses,Production
Mechanism of action
Ferric carboxymaltose is a macromolecular ferric hydroxide carbohydrate complex, which allows for controlled delivery of iron within the cells of the reticuloendothelial system and subsequent delivery to the iron-binding proteins ferritin and transferrin, with minimal risk of release of large amounts of ionic iron in the serum. Intravenous administration of ferric carboxymaltose results in transient elevations in serum iron, serum ferritin and transferrin saturation, and, ultimately, in the correction of haemoglobin levels and replenishment of depleted iron stores.
Side effects
Ferric carboxymaltose was well tolerated, with most drug-related adverse events being mild to moderate in severity. Commonly reported drug-related adverse events included headache, dizziness, nausea, abdominal pain, constipation, diarrhoea, rash and injection site reactions.
The incidence of drug-related adverse events in patients receiving intravenous ferric carboxymaltose was generally similar to that in patients receiving oral ferrous sulfate. In general, rash and local injection-site reactions were more common with ferric carboxymaltose, whereas gastrointestinal adverse events were more frequent with ferrous sulfate. In patients with chronic kidney disease undergoing haemodialysis, a lower proportion of ferric carboxymaltose than iron sucrose recipients experienced at least one drug-related adverse event.
Ferric carboxymaltose Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SNJ Labs Pvt Ltd | +91-9033216399 +91-9824296655 | Gujarat, India | 17 | 58 | Inquiry |
| West Bengal Chemical Industries Ltd. | +91-3340251500 +91-9088750141 | Kolkata, India | 51 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| Global Calcium Pvt Ltd | +91-8050750904 +91-8040554500 | Karnataka, India | 132 | 58 | Inquiry |
| Cleargreens Pharmaceutical Private Limited | +91-9879791802 +91-9579898158 | Gujarat, India | 14 | 58 | Inquiry |
| Emcure Pharmaceuticals Pvt Ltd | +91-2035070000 +91-2040240000 | Maharashtra, India | 5 | 58 | Inquiry |
| Apicore Pharmaceuticals Pvt Ltd | +91-2662267177 +91-2662267166 | Gujarat, India | 181 | 58 | Inquiry |
| Zibo Wei Bin Import & Export Trade Co. Ltd. | +86-0533-2091136 +8613864437655 | China | 100 | 58 | Inquiry |
| Biopole Pharmatech Co., Ltd. | +8615151475053 | China | 37 | 58 | Inquiry |
| airuikechemical co., ltd. | +undefined86-15315557071 | China | 983 | 58 | Inquiry |





