ChemicalBook > Product Catalog >Analytical Chemistry >Standard >Pharmaceutical Impurity Reference Standards >Cefazolin Impurity 9
Cefazolin Impurity 9
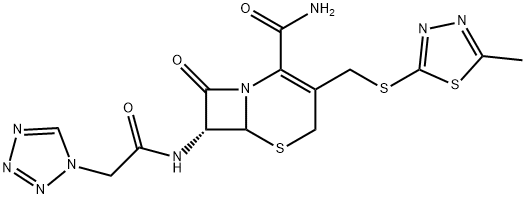
- CAS No.
- 1322626-65-2
- Chemical Name:
- Cefazolin Impurity 9
- Synonyms
- Cefazolin Impurity 9;Cefazolin Impurity 54;Cefazolin Sodium Impurity K;Cefazolin Sodium EP Impurity K;Cefazolin Impurity 10(Cefazolin EP Impurity K);Cefazolin EP Impurity K (Mixture of Diastereomers);5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxamide, 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[2-(1H-tetrazol-1-yl)acetyl]amino]-, (7R)-;(7R)-7-(2-(1H-Tetrazol-1-yl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxamide (Cefazolin Sodium Impurity);Cefazolin impurity 8/Cefazolin EP Impurity K/(7R)-7-(2-(1H-tetrazol-1-yl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
- CBNumber:
- CB83359172
- Molecular Formula:
- C14H15N9O3S3
- Molecular Weight:
- 453.52
- MOL File:
- 1322626-65-2.mol
- Modify Date:
- 2023/5/15 10:44:01
Cefazolin Impurity 9 Preparation Products And Raw materials
Raw materials
Preparation Products
Cefazolin Impurity 9 Suppliers
Global( 31)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Shenzhen Excellent Biomedical Technology Co.,Ltd. | +86-0755-26050679 +86-15915472436 | China | 1031 | 58 | Inquiry |
| Guangzhou PI PI Biotech Inc | +86-020-81716320 +86-13602409664 | China | 3635 | 55 | Inquiry |
| Henan Vcommend Consultrading Co., Ltd. | 13393721940 | China | 10203 | 58 | Inquiry |
| Shanghai Haohong Pharmaceutical Co., Ltd. | 4008210725 4008210725 | China | 55023 | 58 | Inquiry |
| Nantong Hanfang Biotechnology Co. , Ltd. | 18616537568 | China | 30968 | 58 | Inquiry |
| Guangzhou Longying Biotechnology Co., Ltd. | 15975567350 15975567350 | China | 476 | 58 | Inquiry |
| Guangzhou Lanning Biotechnology Co. LTD | +86-15813355568 +86-15813355568 | China | 821 | 58 | Inquiry |
1322626-65-2(Cefazolin Impurity 9)Related Search:
Des[(5-Methyl-1,3,4-thiadiazol-2-yl)thio] Cefazolin-3-Methanol
(6R,7R)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-7-pivalamido-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Cefazolin sodium salt
Cefazolin impurity B
Cefazolin IMpurity C
(6R-trans)-3-(acetoxymethyl)-8-oxo-7-(1H-tetrazol-1-ylacetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
sodium (6R-trans)-3-(acetoxymethyl)-8-oxo-7-(1H-tetrazol-1-ylacetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
7-Amino-3-[(5-methyl-1,3,4-thiadiazol-2-ylthio)methyl]-3-cephem-4-carboxylic Acid
7-Aminocephalosporanic acid
Cefazolin SodiuM iMpurity G
chevron_left
1of3
chevron_right
Cefazolin Sodium EP Impurity K
Cefazolin impurity 8/Cefazolin EP Impurity K/(7R)-7-(2-(1H-tetrazol-1-yl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Cefazolin Impurity 10(Cefazolin EP Impurity K)
Cefazolin Impurity 9
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxamide, 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[2-(1H-tetrazol-1-yl)acetyl]amino]-, (7R)-
Cefazolin Impurity 54
Cefazolin Sodium Impurity K
(7R)-7-(2-(1H-Tetrazol-1-yl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxamide (Cefazolin Sodium Impurity)
Cefazolin EP Impurity K (Mixture of Diastereomers)
1322626-65-2





