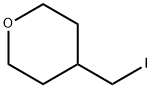
4-(Iodomethyl)tetrahydro-2H-pyran
- CAS No.
- 101691-94-5
- Chemical Name:
- 4-(Iodomethyl)tetrahydro-2H-pyran
- Synonyms
- 4-(iodomethyl)oxane;4-IODOMETHYLTETRAHYDROPYRAN;4-(Iodomethyl)tetrahydropyrane;4-(IODOMETHYL)TETRAHYDRO-2H-PYRAN;(4-TETRAHYDROPYRANYL)METHYL IODIDE;Tetrahydro-4-(iodomethyl)-2H-pyran;4-(Iodomethyl)tetrahydro-2H-pyrane;2H-Pyran, tetrahydro-4-(iodomethyl)-
- CBNumber:
- CB9120260
- Molecular Formula:
- C6H11IO
- Molecular Weight:
- 226.06
- MOL File:
- 101691-94-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/11/21 9:27:28
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P260-P264-P280-P301+P310+P330+P331-P303+P361+P353+P310-P304+P340+P310-P305+P351+P338+P310-P363-P405-P501 | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 23-26-36/37/39-45 | |||||||||
| NFPA 704 |
|
4-(Iodomethyl)tetrahydro-2H-pyran Chemical Properties,Uses,Production
Synthesis
4-(Iodomethyl)tetrahydro-2H-pyran is prepared from 4-(methanesulfonyloxymethyl)tetrahydropyran by heating and refluxing under conditions of reaction with sodium iodide in acetone. The specific method of operation is described below:
.
Preparation: A mixture of Preparation (328g, 1.69mol) and sodium iodide (507g, 3.4mol) in acetone (3.3L) was refluxed for 4h. TLC (diethyl ether) showed significant mesylate remaining so further sodium iodide (127g, 0.65mol) was added and reflux continued for 16h. The mixture was cooled and filtered. The resulting cake was washed with acetone, dried, and11then partitioned between diethyl ether (800mL) and water (800mL). The aqueous phase was re-extracted with diethyl ether (200mL), the ether extracts combined and washed with 10% sodium thiosulphate solution (300mL) which decolourised the extract. Final washing with water (300mL), drying (MgS04) and then removal of the solvent provided the 4-(IODOMETHYL)TETRAHYDRO-2H-PYRAN (365g, 92% yield).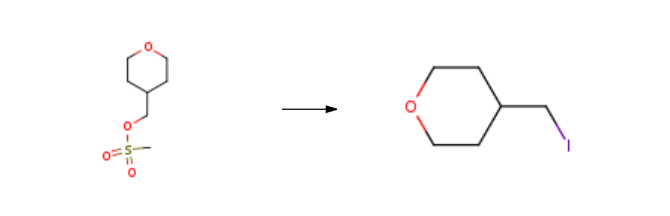
4-(Iodomethyl)tetrahydro-2H-pyran Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Shanghai Boc Chemical Co., Ltd. | 021-34975602; +8618721111801 | China | 138 | 58 | Inquiry |
| Guangzhou Yuheng Pharmaceutical Technology Co., Ltd | +8613580539051 | CHINA | 21149 | 58 | Inquiry |
| Alfa Chemistry | United States | 2344 | 58 | Inquiry | |
| Aston Chemical | 13000000000 | United States | 1634 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| A.J Chemicals | 58 |
| CHEMSWORTH | 30 |
| Clearsynth Labs | 58 |
| career henan chemical co | 58 |
| Chongqing Chemdad Co., Ltd | 58 |
| CONIER CHEM AND PHARMA LIMITED | 58 |
| Shanghai Boc Chemical Co., Ltd. | 58 |
| Guangzhou Yuheng Pharmaceutical Technology Co., Ltd | 58 |
| Alfa Chemistry | 58 |
| Aston Chemical | 58 |





