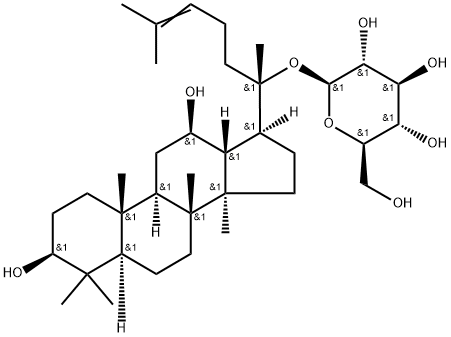
Ginsenoside CK
- CAS No.
- 39262-14-1
- Chemical Name:
- Ginsenoside CK
- Synonyms
- IH-901;CoMpoundCK;Ginsenoside K;Ginsenosinde CK;20S-Ginsenoside C;S-Ginsenoside C-K;GINSENOSIDE COMPOUND K(P);Compound K-Ginsenoside CK;Lappaconitine hydrochloride;Ginsenoside Compound K >=96% (HPLC)
- CBNumber:
- CB92332311
- Molecular Formula:
- C36H62O8
- Molecular Weight:
- 622.88
- MOL File:
- 39262-14-1.mol
- Modify Date:
- 2024/7/19 19:28:24
| Melting point | 181~183℃ |
|---|---|
| Boiling point | 723.1±60.0 °C(Predicted) |
| Density | 1.19 |
| solubility | DMF: 10 mg/ml; DMSO: 10 mg/ml; DMSO:PBS (pH 7.2) (1:1): 0.5 mg/ml |
| pka | 12.94±0.70(Predicted) |
| form | powder |
| color | White |
| Stability | Hygroscopic |
| InChIKey | FVIZARNDLVOMSU-SFEJUJENNA-N |
| LogP | 5.500 (est) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312+P330-P501 | |||||||||
| NFPA 704 |
|
Ginsenoside CK Chemical Properties,Uses,Production
Description
Ginsenoside compound K (C-K) is a metabolite of the protopanaxadiol-type saponins of Panax ginseng C.A. Meyer, has long been used to treat against the development of cancer, inflammation, allergies, and diabetes; C-K acts as a unique HUVEC migration inhibitor by regulating MMP expression, as well as the activity of SPHK1 and its related sphingolipid metabolites. C-K exhibits anti-inflammatory effects by reducing iNOS and COX-2, C-K exhibits an inhibition against the activity of CYP2C9 and CYP2A6 in human liver microsomes with IC50s of 32.0±3.6 μM and 63.6±4.2 μM, respectively. C-K promotes Aβ clearance by enhancing autophagy via the mTOR signaling pathway in primary astrocytes.
Uses
Ginsenoside C-K is reported to exhibit anti-wrinkle effects. Also, it potentiates tumor necrosis factor (TNF)-related apotosis-inducing ligand (TRAIL)-induced apotosis in HCT116 colon cancer.
General Description
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate.
Biochem/physiol Actions
Ginsenoside compound K (GCK, 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol, C36H62O8, also known as M1, IH-901, Ginsenoside CK), belonging to tetracyclic dammarane-type triterpenoid saponins. Ginsenosides are poorly absorbed from the gut by oral administration. Still, their major intestinal bacterial metabolite GCK is absorbed, indicating that the in vivo action of ginsenosides oral administration is mediated by their intestinal bacterial metabolic component GCK. The various processes used for GCK synthesis include enzymatic use, microbial conversion, heating, mycelial fermentation, and metabolic engineering[1-3].
Mechanism of action
Mechanistically, Ginsenoside CK (CK) was found to inhibit hormone-independent breast cancer growth by decreasing cyclin D1 expression to induce G1 cycle arrest and induce apoptosis in MCF-7 human breast cancer cells by activating adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) through the production of reactive oxygen species (ROS). High glutamine-addicted TNBC cells were particularly sensitive to CK treatment. Ginsenoside CK exerted antitumor activity against TNBC by suppressing glutamine consumption and glutamate production via downregulation of glutaminase 1 (GLS1) expression. CK treatment further decreased cellular ATP production, reduced the utilisation of amino acids associated with glutamine metabolism, and induced glutathione (GSH) depletion and reactive oxygen species (ROS) accumulation, consequently triggering apoptosis in TNBC. Furthermore, CK decreased GLS1 expression in SUM159 xenograft mouse mammary tumors and significantly inhibited tumor growth with few side effects[2].
References
[1] Anshul Sharma, Hae-Jeung Lee. “Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities.” Biomolecules (2020).
[2] Bo Zhang. “Ginsenoside CK induces apoptosis in triple-negative breast cancer cells by targeting glutamine metabolism.” Biochemical pharmacology 1 1 (2022): 115101.
[3] Mengshi Tang . “Ginsenoside compound K- a potential drug for rheumatoid arthritis.” Pharmacological research 166 (2021): Article 105498.
Ginsenoside CK Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | China | 2971 | 58 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | China | 3777 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | China | 2637 | 58 | Inquiry |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | China | 1000 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16924 | 58 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9337 | 55 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
39262-14-1(Ginsenoside CK)Related Search:
1of4
chevron_right




