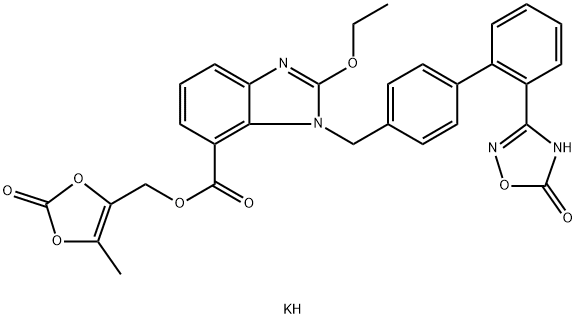
Azilsartan kaMedoxoMil
- CAS No.
- 863031-24-7
- Chemical Name:
- Azilsartan kaMedoxoMil
- Synonyms
- TAK 491 kamedoxomil;TAK-491 monopotassium;Azilsartan kaMedoxoMil;Azilsartan KaModoxoMil;Azilsartan KaMedoximil;Azilsartan Mehtyl ester;Azilsartan MedoxMil PotassuiM;Azilsartan Medoxomil Potassium;Azilsartan MedoxiMil PotassiuM;Azilsartan MedoxoMil PotassiuM salt
- CBNumber:
- CB92549391
- Molecular Formula:
- C30H25KN4O8
- Molecular Weight:
- 608.65
- MOL File:
- 863031-24-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/7 19:09:42
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Azilsartan kaMedoxoMil Chemical Properties,Uses,Production
Definition
ChEBI: An organic potassium salt that is the monopotassium salt of azilsartan medoxomil. A prodrug for azilsartan, it is used for treatment of hypertension.
Clinical Use
Azilsartan kamedoxomil, developed by Takeda Pharmaceuticals, was approved for the treatment of hypertension and launched in the U.S. under the brand name Edarbi. Edarbi is a prodrug that undergoes rapid hydrolysis to liberate azilsartan, the active ingredient (TAK-536, 39). As the 8th angiotensin receptor blocker (ARB) to enter the world market, azilsartan kamedoxomil can function as monotherapy or in combination with other antihypertensive agents. In several clinical studies, monotherapeutic azilsartan kamedoxomil showed superior antihypertensive activity and a favorable safety/tolerability profile in patients compared with other established therapeutics, including valsartan, olmesartan medoxomil, candesartan, and telmisartan.In late 2011,Takeda announced that FDA also approved the fixed-dose combination tablet of azilsartan kamedoxomil with chlorthalidone under the trade name of Edarbyclor.
Synthesis
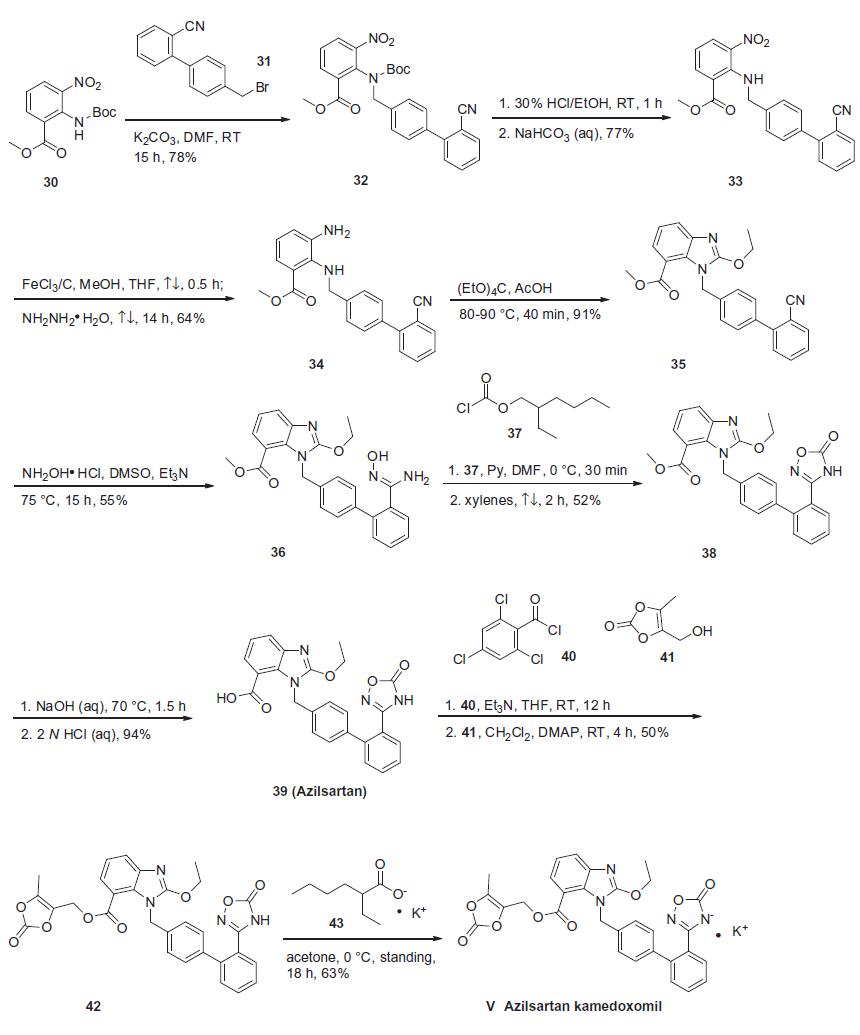
Based on the synthesis of azilsartan, the process-scale approach to azilsartan kamedoxomil is described in the scheme. The synthesis started with commercially available methyl 2-[(tert-butoxycarbonyl)amino]-3-nitrobenzoate (30), which can also be prepared by several different routes.41,42 Alkylation of 30 with diaryl bromide 31 gave benzylamine 32 in 78% yield, which was followed by deprotection with 30% ethanolic HCl and alkalinization to produce amine 33 in 77% yield. The nitro group within 33 was reduced with hydrazine hydrate and a catalytic amount of ferric chloride to afford 2,3-diaminobenzoate 34 in 64% yield. Ring formation was achieved by treatment of 34 with tetraethoxymethane and acetic acid to produce benzimidazole 35 in 91% yield.37 The addition of hydroxylamine to the cyano group of 35 provided amidoxime 36 in 55% yield, which underwent immediate cyclization upon treatment with 2-ethylhexyl chloroformate 37 in refluxing xylenes to give oxadiazolone 38 in 52% yield. Hydrolysis of 38 gave azilsartan (39, TAK-536) in 94% yield. In the presence of perchlorobenzoyl chloride 40 and triethylamine, carboxylic acid 39 was converted to the mixed acid anhydride intermediate, which when condensed with alcohol 41 furnished benzoate 42 in 50% yield. Salt preparation of 42 was accomplished with potassium 2-ethylhexyl carboxylate 43 affording azilsartan kamedoxomil (V) in 63% yield.
Azilsartan kaMedoxoMil Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 461 | 58 | Inquiry |
| Metrochem API Private Limited | +91-4069069999 +91-4069069999 | Telangana, India | 78 | 58 | Inquiry |
| Aurobindo Pharma Limited | +914066725000 | Telangana, India | 112 | 58 | Inquiry |
| Piramal Pharma Solutions | +919892735994 | Maharashtra, India | 48 | 58 | Inquiry |
| Alembic Pharmaceuticals Limited | +91-2652280880 +91-2652280550 | Gujarat, India | 115 | 58 | Inquiry |
| Honour Lab Limited | +919845977466 | Telangana, India | 164 | 58 | Inquiry |
| CTX Lifesciences Pvt Ltd | +91-2612399669 +91-7434851395 | Gujarat, India | 28 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| Afton Pharma | +91-9712000202 +91-9712000202 | Gujarat, India | 63 | 58 | Inquiry |
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |





