Azilsartan Medoxomil
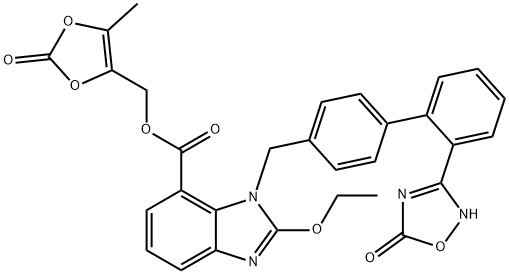
- CAS No.
- 863031-21-4
- Chemical Name:
- Azilsartan Medoxomil
- Synonyms
- TAK 491;Azilsartan MedoxiMil;CS-306;Azizartan ester;Azilsaran medoxomil;Azilsartan medoxomil;Azilsartan MedoxoMil(TAK 491);Azilsartan Kamedoxomil Form I;Azilsartan Medoxomil USP/EP/BP;Azilsartan Acid Medoxomil Ester
- CBNumber:
- CB32516566
- Molecular Formula:
- C30H24N4O8
- Molecular Weight:
- 568.53
- MOL File:
- 863031-21-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/7/5 17:39:42
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| NFPA 704 |
|
Azilsartan Medoxomil Chemical Properties,Uses,Production
Description
Azilsartan medoxomil (Edarbi), an angiotensin II receptor antagonist, was approved by the U.S. FDA in February 2011 for the treatment of hypertension in adults. The discovery of azilsartan was the result of a medicinal chemistry effort to identify an ARB with a different carboxylic acid isostere than the ones found in the marketed ARBs. Several of the marketed ARBs use a tetrazole group as a carboxylic acid isostere. The medicinal chemistry approach that led to azilsartan involved the replacement of this commonly used tetrazole with a 5-oxo-1,2,4-oxadiazole group. Azilsartan can be synthesized by Suzuki coupling of p-tolyl boronic acid to 2-bromobenzonitrile, followed by bromination of the methyl group. The bromide is displaced to introduce a protected 2-ethoxy-1H-benzo[d] imidazole-7-carboxylate. The cyano group is converted to a hydroxylamidine, followed by reaction with an alkyl-chloroformate and intramolecular cyclization to form the 5-oxo-1,2,4-oxadiazole ring. The acid is then deprotected and converted to a prodrug. The parent, azilsartan has been extensively characterized in vitro and compared with other marketed AT1 antagonists olmesartan, valsartan, telmisartan, irbesartan, and candesartan. Azilsartan was found to be a potent (IC50=2.6 nM), selective, inverse agonist of the AT1 receptor. From washout experiments, azilsartan was found have slow dissociation from the receptor and thus is characterized as an insurmountable antagonist.
Definition
ChEBI: A carboxylic ester obtained by formal condensation of the carboxy group of azilsartan with the hydroxy group of 4-(hydroxymethyl)-5-methyl-1,3-dioxol-2-one. A prodrug for azilsartan, it is used for treatment of hypertension.
Azilsartan Medoxomil Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SGMR PHARMACEUTICALS PVT LTD | +91-9032001889 +91-9032001889 | Telangana, India | 83 | 58 | Inquiry |
| ALMELO PRIVATE LIMITED | +91-9100991345 +91-8500807769 | Telangana, India | 114 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| BDR Pharmaceuticals International Pvt Ltd | +91-2240560560 +91-7718884418 | Maharashtra, India | 206 | 58 | Inquiry |
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |
| Sibram Pharmaceutical | +91-8655777550 +91-8655777550 | Maharashtra, India | 244 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| R.B.Chemical | 08068442240Ext 273 | Gujarat, India | 8 | 58 | Inquiry |
| Seine Pharma Private Limited | 08069033811Ext 842 | Hyderabad, India | 78 | 58 | Inquiry |
863031-21-4(Azilsartan Medoxomil)Related Search:
1of4
chevron_right




