Bedaquiline fumarate
- CAS No.
- 845533-86-0
- Chemical Name:
- Bedaquiline fumarate
- Synonyms
- Badaquiline Fumarate;R 403323;TMC207 fumarate;R207910 fumarate;Sirturo fumarate;Bedaquiline Fumarat;Bedaquiline (fuMarate);TMC-207;TMC207;TMC 207;Bedaquinoline fumarate;Fumaric acid bedaquiline
- CBNumber:
- CB92705076
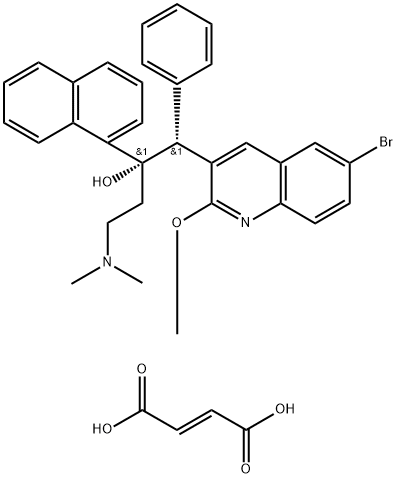
- Molecular Formula:
- C36H35BrN2O6
- Molecular Weight:
- 671.59
- MOL File:
- 845533-86-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/3/27 6:45:46
| storage temp. | Inert atmosphere,Room Temperature |
|---|---|
| solubility | DMSO : 100 mg/mL (148.90 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) |
| form | Powder |
| color | White to off-white |
| InChIKey | ZLVSPMRFRHMMOY-KZDJUSSONA-N |
| SMILES | C(/C(=O)O)=C\C(=O)O.[C@](C1=CC=CC2C=CC=CC1=2)(O)(CCN(C)C)[C@H](C1C=CC=CC=1)C1=CC2C=C(Br)C=CC=2N=C1OC |&1:8,25,r| |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 |
Bedaquiline fumarate Chemical Properties,Uses,Production
Description
Bedaquiline fumarate (BQF) is an FDA-approved antituberculosis drug that targets the enzyme ATP synthase. It is a fumarate salt prepared from equimolar amounts of bedaquiline and fumaric acid. It can be used in combination therapy for the treatment of multidrug-resistant tuberculosis in the lungs of adults (18 years of age and older). The new BQF microemulsion dosage form of BQF has improved oral bioavailability over the previous formulation, and the BQF microemulsion is cytocompatible with significantly higher cellular uptake than the control group at the highest concentration of 500 μg/ml, which could lead to further use in the effective treatment of multidrug-resistant tuberculosis[1].
Characteristics
Bedaquiline fumarate is the first diarylquinoline analogue used in the treatment of M. tuberculosis and its main biologically active form is bedaquiline. Bedaquiline fumarate forms three degradation products associated with fumaric acid (DP1, DP2 and DP3) under photolysis conditions and the demethylation product DP4 under acidic conditions. Bedaquiline forms four degradation products associated with tertiary alcohols and tertiary amine moiety side chains (IM1, IM2, IM3, and IM4) under photolysis conditions, and DP4 under acidic conditions.Both compounds are stable under thermal, alkaline and oxidative conditions. DP1 and DP2 show that fumarates can undergo cis-trans isomerisation under light conditions. The fumarate form stabilises the side chain of bedaquiline. It is advisable to avoid contact with acids and light during storage and production[2].
Definition
ChEBI: Bedaquiline fumarate is a fumarate salt prepared from equimolar amounts of bedaquiline and fumaric acid. It is used in combination therapy for the treatment of pulmonary multi-drug resistant tuberculosis by inhibition of ATP synthase, an enzyme essential for the replication of the mycobacteria. It has a role as an antitubercular agent and an ATP synthase inhibitor. It contains a bedaquiline(2+).
Clinical Use
Bedaquiline fumarate is a diarylquinone drug developed by Janssen Pharmaceutical which is marketed under the trade name Sirturo ®. The drug, which was approved in 2012 for the treatment of multidrug-resistant tuberculosis (MDR-TB), was developed in partnership with Johnson & Johnson and represents the first new tuberculosis therapy approved in over four decades. Bedaquiline is the first member of a new class of diarylquinone compounds whose mechanism of action inhibits Mycobaterium tuberculosis ATP synthase which deprives bacterium of energy.
Synthesis
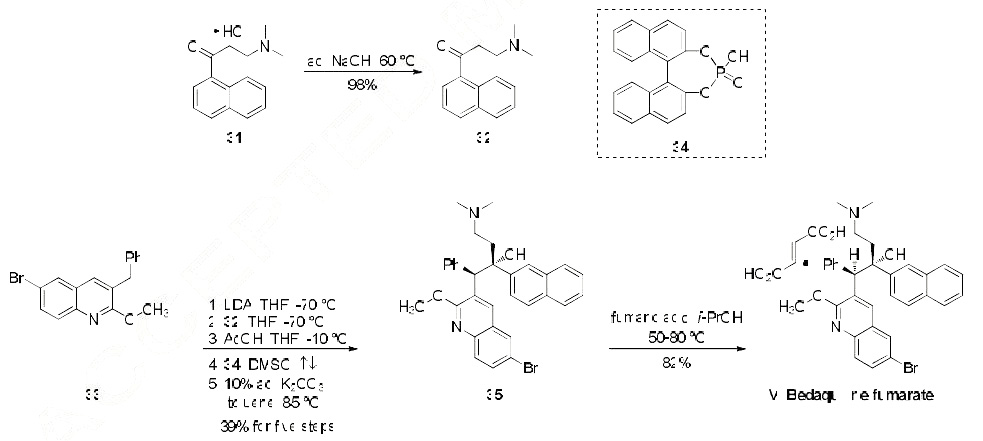
Of the relatively few synthetic approaches to bedaquiline (or its fumarate salt) that have been reported, the most likely process-scale route is that described by Porstmann and co-workers from Janssen Pharmaceutical, and this route is outlined in the scheme. The synthesis was initiated by first freebasing commercially available dimethylaminoketone 31 with sodium hydroxide to provide naphthylone 32 in nearly quantitative yield. Subjection of commercially available quinoline 33 to LDA removed the benzyllic proton within this system and subsequent trap with naphthylone 32 gave rise to a mixture of diastereomers whereby the major diastereomer obtained from this reaction corresponded to the bedaquiline geometry. The minor diastereomer was resolved through multiple recrystallizations and seeding techniques. This racemate of the major diastereomer subsequently underwent a chiral resolution upon treatment with BINAP derivative 34 in refluxing DMSO and then upon cooling and subjection to aqueous base in warm toluene furnished bedaquiline 35 bearing the requisite (R,S)- configuration of the two vicinal chiral centers corresponding to that of the drug. The overall yield of the conversion of 33 to enantiopure 35 was 39%. Aminoquinolinol 35 was then prepared as the corresponding fumarate salt upon treatment with fumaric acid in the presence of isopropanol, and this salt formation delivered bedaquiline fumarate (VI) in 82% yield.
References
[1] VISHWAS P PARDHI. Bedaquiline fumarate microemulsion: formulation optimization, rheological characterization and in vitro studies.[J]. Nanomedicine (London, England), 2022, 17 21: 1529-1546. DOI:10.2217/nnm-2022-0132.
[2] KAIJING GUO; Chen M; Yanan Wang. Characterization of stress degradation products of bedaquiline fumarate and bedaquiline by LC-PDA and LC-MS[J]. Journal of pharmaceutical and biomedical analysis, 2023. DOI:10.1016/j.jpba.2023.115658.
Bedaquiline fumarate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Arene Lifesciences Limited | +91-9849847907 +91-8455241148 | Telangana, India | 55 | 58 | Inquiry |
| MSN LIFE SCIENCES PRIVATE LTD | +91 84523 34200 | New Delhi, India | 104 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Hema Pharmaceuticals Pvt. Ltd. | 08048250474Ext 875 | Gujarat, India | 24 | 58 | Inquiry |
| Chengdu Aupone Pharmaceutical Co.Ltd. | +86-28-+86-28-87843998-6060-6060 +8618631098571 | China | 55 | 58 | Inquiry |
| Shanghai Famo Biotech Co Ltd | +86-36680037 +86-18550473860 | China | 528 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20235 | 58 | Inquiry |





