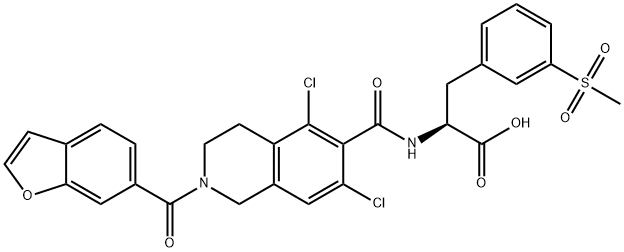
lifitegrast
- CAS No.
- 1025967-78-5
- Chemical Name:
- lifitegrast
- Synonyms
- Lifitegrast-d4;liftegrast;Lifitegrast Impurity;CS-2633;(S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoic acid;Amipak;SHP-606;SAR 1118;Rituxite;Litastat
- CBNumber:
- CB92738258
- Molecular Formula:
- C29H24Cl2N2O7S
- Molecular Weight:
- 615.48
- MOL File:
- 1025967-78-5.mol
- Modify Date:
- 2025/4/28 21:36:36
| Melting point | >163°C (dec.) |
|---|---|
| Boiling point | 811.9±65.0 °C(Predicted) |
| Density | 1.479±0.06 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Sonicated) |
| form | Solid |
| pka | 3.14±0.10(Predicted) |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | JFOZKMSJYSPYLN-QHCPKHFHSA-N |
| SMILES | C(O)(=O)[C@H](CC1=CC=CC(S(C)(=O)=O)=C1)NC(C1C(Cl)=CC2=C(C=1Cl)CCN(C(C1C=C3OC=CC3=CC=1)=O)C2)=O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
lifitegrast Chemical Properties,Uses,Production
Description
Lifitegrast (Xiidra; lifitegrast sodium; SAR1118; SHP606; SPD606) is a lymphocyte functionassociated antigen-1 (LFA-1) antagonist. It inhibits T-cell inflammation by blocking the binding of two key surface proteins (LFA-1 and ICAM-1) that mediate the chronic inflammatory cascade associated with dry eye disease. In a phase III clinical trial, lifitegrast 5% ophthalmic solution (50 mg/mL) is administered as a single 0.2mL eye drop twice a day into each eye for an 84 day treatment period.

Lifitegrast does not currently have Marketing Authorisation in the EU for any indication. Lifitegrast is licensed for use in the USA for treatment of the signs and symptoms of dry eye disease. The most common adverse reactions (incidence 5-25%) reported following the use of lifitegrast are instillation site irritation, dysgeusia, and decreased visual acuity.
Chemical Properties
Lifitegrast is a white to off-white powder which is soluble in water.
Uses
Lifitegrast is used for the treatment of signs and symptons of dry eye diseases. It also Inhibites corneal inflammation that is capable of causing pains, blurred vision and ocular discomfort in sufferer.
Indications
Topical lifitegrast was approved by the FDA for the treatment of dry eye. Lifitegrast decreases inflammation by blocking the interaction between intercellular adhesion molcule 1 and lymphocyte function- -associated antigen 1. In four, large, multicellular, randomized clinical trials, lifitegrast was shown to be effective in improving the signs and symptoms of dry eye. The side effects of lifitegrast include transient ocular irritation and dysgeusia. Further studies are needed to explore the effectiveness of combination therapy such as the concomitant use of topical cyclosporine and topical liftegrast.
Definition
ChEBI: An N-acyl-L-alpha-amino acid obtained by formal condensation of the carboxy group of N-[2-(1-benzofuran-6-carbonyl)]-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid with he amino group of 3-(methanesulfonyl)-L-phenylalanine. Used for treatment of keratoconjunctivitis sicca (dry eye syndrome).
Mechanism of action
Lifitegrast binds to the integrin lymphocyte function-associated antigen-1 (LFA-1), a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular adhesion molecule-1 (ICAM-1). ICAM-1 may be overexpressed in corneal and conjunctival tissues in dry eye disease. LFA-1/ICAM-1 interaction can contribute to the formation of an immunological synapse resulting in T-cell activation and migration to target tissues. In vitro studies demonstrated that lifitegrast may inhibit T-cell adhesion to ICAM-1 in a human T-cell line and may inhibit secretion of inflammatory cytokines in human peripheral blood mononuclear cells. The exact mechanism of action of lifitegrast in dry eye disease is not known.
Pharmacokinetics
In a subset of dry eye disease patients (n=47) enrolled in a Phase 3 trial, the pre-dose (trough) plasma concentrations of lifitegrast were measured after 180 and 360 days of topical ocular dosing (1 drop twice daily) with Xiidra (lifitegrast ophthalmic solution) 5%. A total of nine (9) of the 47 patients (19%) had plasma lifitegrast trough concentrations above 0.5 ng/mL (the lower limit of assay quantitation). Trough plasma concentrations that could be quantitated ranged from 0.55 ng/mL to 3.74 ng/mL.
Side effects
A multicenter, randomized, double-masked, placebo-controlled phase 3 study (n = 331) evaluating the safety of lifitegrast ophthalmic solution for the treatment of dry eye disease reported the most common non-ocular effect was dysgeusia (change in taste) occurring in 16.4% of patients in the lifitegrast group and 1.8% of the placebo group.
References
[1] Stacy L. Haber. “Lifitegrast: a novel drug for patients with dry eye disease.” ACS Applied Bio Materials (2019).
lifitegrast Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| Honour Lab Limited | +919845977466 | Telangana, India | 164 | 58 | Inquiry |
| Mankind Pharma Limited | +91-1146541111 +91-1146541111 | New Delhi, India | 23 | 58 | Inquiry |
| Precise Biopharma Pvt Ltd | +91-2267828600 +91-2267828600 | Maharashtra, India | 46 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| SUN PHARMACEUTICAL INDUSTRIES LTD | (+91 22) 4324 4324 | New Delhi, India | 175 | 58 | Inquiry |
| MSN LIFE SCIENCES PRIVATE LTD | +91 84523 34200 | New Delhi, India | 104 | 58 | Inquiry |
| AUROBINDO PHARMA LTD | +91 (40) 6672 1200 | New Delhi, India | 209 | 58 | Inquiry |
| DR REDDYS LABORATORIES LTD | +91-40-49002900 | New Delhi, India | 201 | 58 | Inquiry |
| GLENMARK LIFE SCIENCES LTD | +91 22 4018 9999 | New Delhi, India | 103 | 58 | Inquiry |
1025967-78-5(lifitegrast)Related Search:
1of2
chevron_right




