Beryllium Peroxyphosphate
- CAS No.
- Chemical Name:
- Beryllium Peroxyphosphate
- Synonyms
- Beryllium Peroxyphosphate
- CBNumber:
- CB94865927
- Molecular Formula:
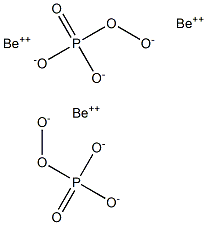
- Be3O10P2
- Molecular Weight:
- 248.978068
- MOL File:
- Mol file
Beryllium Peroxyphosphate Chemical Properties,Uses,Production
Description
The chemistry of beryllium phosphates is far from
complete. Beryllium peroxyphosphate has never been
prepared. The chemistry involved and the physical
properties of these compounds remain little known.
Only a few references in the literature are available
and most of these are dated before 1900. The compounds
were identified by analysis, i.e. %Be, %P and %O as an
oxidant. Parsons wrote a monograph “The Chemistry
of Beryllium and Its Compounds” in 1910 but had little
to add to what was already known.
Beryllium peroxyphosphates could be prepared by
both solid-state or solution methods. However, it is likely
that the two sets of compounds would differ. For the
monoperoxyphosphates, one would get Be(HPO5)2 for
the former method and (BeOH)(HPO5) for an aqueous
solution. The question of hydrates remains unknown.
In the solution method, one could use the acid, H3PO5
or the potassium salt K2HPO5. Whether the solution
should be acidic or basic is not known. Like the other
alkaline earths, it is probably best to prepare the acid
in a nonaqueous solution like carbon tetrachloride and
;then add the potassium compound for the basic solution:
P2O5 + 2H2O2 +H2O ? 2H3PO5
2H3PO5 + KOH ? K2HPO5





