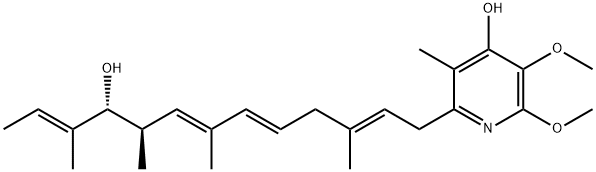
PIERICIDIN A
- CAS No.
- 2738-64-9
- Chemical Name:
- PIERICIDIN A
- Synonyms
- SN 198E;IT-143D;piericidin;piericidinea;PIERICIDIN A;PIERICIDIN A1;SHAOGUAMYCIN B;SHAOGUANMYCIN B;piericidin(sub1);piericidin a from microbial source
- CBNumber:
- CB9758229
- Molecular Formula:
- C25H37NO4
- Molecular Weight:
- 415.57
- MOL File:
- 2738-64-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/8 9:02:15
| Boiling point | 614.9±55.0 °C(Predicted) |
|---|---|
| Density | 1.044±0.06 g/cm3(Predicted) |
| Flash point | 85 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 20 mg/ml). |
| pka | 5.21±0.33(Predicted) |
| form | liquid |
| color | green-yellow |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or Ethanol may be stored at -20°C for up to 1 month. |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Hazard Codes | T+,Xn | |||||||||
| Risk Statements | 26/27/28-20/21/22 | |||||||||
| Safety Statements | 28-36/37-45 | |||||||||
| RIDADR | UN 3382 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YD4588000 | |||||||||
| F | 3-10 | |||||||||
| HS Code | 2933399990 | |||||||||
| NFPA 704 |
|
PIERICIDIN A Chemical Properties,Uses,Production
Uses
Piericidin A is the major analogue of a family of pyridyl antibiotics isolated from selected Streptomyces species. It is a specific, potent inhibitor of NADH-ubiquinone oxidoreductase (Complex I) that binds to ubiquinone binding site(s). Piericidin A inhibits both mitochondrial and bacterial NADH-ubiquinone oxidoreductases, binding close to NUOD-NUOB interface.
Biochem/physiol Actions
Piericidin A (PA), an insecticidal metabolite of Streptomyces mobaraensis is a potential quorum-sensing inhibitors (QSIs) that can destroy the expression of the virulence genes of Erwinia carotovora subsp. atroseptica (Eca). Hence it may be used as control agents of soft rot disease on potato tubers.
Enzyme inhibitor
This reduced antibiotic (FW = 423.64 g/mol; CAS 2738-64-9; Soluble in ethanol, methanol, DMF or DMSO) is a structural analogue of ubiquinone and a partially competitive inhibitor of bacterial and mitochondrial Type-I NADH-ubiquinone oxidoreductases (or Complex I). Octahydropiericidin A also inhibits glucose dehydrogenase.
PIERICIDIN A Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Alfa Chemistry | +1-5166625404 | United States | 21317 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Shanghai Aladdin Biochemical Technology Co.,Ltd. | +86-18521732826 | China | 48467 | 58 | Inquiry |
| Hangzhou Huiyi Biotechnology Co., LTD | 0571-89918262 13357170655 | China | 236 | 58 | Inquiry |
| Shanghai?Medlife?Pharm-Tech?Co.,?Ltd | 021-59167510 18117107507 | China | 5014 | 58 | Inquiry |
| Focus Biomolecules | 855-362-8721 | United States | 1284 | 58 | Inquiry |





