ChemicalBook > Product Catalog >Biochemical Engineering >Amino Acids and Derivatives >Phenylalanine derivatives >Fmoc-p(NH-Boc)-L-Phe-OH
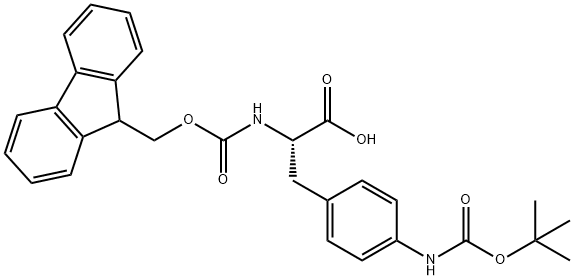
Fmoc-p(NH-Boc)-L-Phe-OH
- CAS No.
- 174132-31-1
- Chemical Name:
- Fmoc-p(NH-Boc)-L-Phe-OH
- Synonyms
- Fmoc-L-4-aminophe;RARECHEM BK PT 0176;FMOC-PHE(4-NHBOC)-OH;FMOC-PHE(P-NHBOC)-OH;FMOC-L-PHE(4-NH-BOC);FMOC-L-PHE(4-NHBOC)-OH;FMOC-L-4-AMINOPHE(BOC);Fmoc-Phe(Boc-4-NH2)-OH;M Fmoc-Phe(4-NHBoc)-OH;FMOC-P(NH-BOC)-L-PHE-OH
- CBNumber:
- CB9853203
- Molecular Formula:
- C29H30N2O6
- Molecular Weight:
- 502.56
- MOL File:
- 174132-31-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:42
| Boiling point | 671.0±55.0 °C(Predicted) |
|---|---|
| Density | 1.280±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| form | Solid |
| pka | 3.80±0.10(Predicted) |
| CAS DataBase Reference | 174132-31-1(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29225090 | |||||||||
| NFPA 704 |
|
Fmoc-p(NH-Boc)-L-Phe-OH Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 129)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26216 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | China | 19915 | 58 | Inquiry |
| Labnetwork lnc. | +86-27-50766799 +8618062016861 | China | 19994 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| A.J Chemicals | 58 |
| Capot Chemical Co.,Ltd. | 60 |
| CONIER CHEM AND PHARMA LIMITED | 58 |
| career henan chemical co | 58 |
| Career Henan Chemica Co | 58 |
| Dideu Industries Group Limited | 58 |
| BOC Sciences | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 58 |
| Nextpeptide Inc | 58 |
| Labnetwork lnc. | 58 |
174132-31-1(Fmoc-p(NH-Boc)-L-Phe-OH)Related Search:
FMOC-4-AZIDO-L-PHENYLALANINE
FMOC-L-4-THIAZOLYLALANINE
(S)-N-Fmoc-4-Bromophenylalanine
FMOC-L-3,5-DIFLUOROPHE
Fmoc-L-4-Iodophenylalanine
FMOC-L-4-Trifluoromethylphe
FMOC-L-4-CYANOPHENYLALANINE
FMOC-L-PHE(4-COCH3)
FMOC-D, L-PHE(4-CH2NH-BOC)
FMOC-BPA-OH
chevron_left
1of4
chevron_right
3-(4-TERT-BUTOXYCARBONYLAMINO-PHENYL)-2-(9H-FLUOREN-9-YLMETHOXYCARBONYLAMINO)-PROPIONIC ACID
4-Amino-L-phenylalanine, N-FMOC protected
RARECHEM BK PT 0176
(S)-FMOC-(4-BOC-AMINO)PHENYLALANINE
N-ALPHA-FMOC-L-4-(BOC-AMINO)-PHENYLALANINE
N-ALPHA-(9-FLUORENYLMETHOXYCARBONYL)-4-AMINO-N-T-BUTOXYCARBONYL-L-PHENYLALANINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-4-TERT-BUTYLOXYCARBONYLAMINO-L-PHENYLALANINE
FMOC-PHE(4-NHBOC)-OH
FMOC-P(NH-BOC)-L-PHE-OH
FMOC-PHE(P-NHBOC)-OH
FMOC-P-AMINO-PHE(BOC)-OH
FMOC-P-AMINO-PHENYLALANINE(BOC)-OH
FMOC-4-(BOC-AMINO)-L-PHENYLALANINE
FMOC-D, L-PHE(4-NH-BOC)
FMOC-(BOC-4-AMINO)-L-PHENYLALANINE
FMOC-L-PHE(4-NH-BOC)
FMOC-L-PHE(4-NHBOC)-OH
FMOC-L-4-AMINOPHE(BOC)
FMOC-L-4-AMINOPHENYLALANINE(BOC)
Fmoc-L-4-Amino-phe(Boc)-OH
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-4-T-BUTYLOXYCARBONYLAMINO-L-PHENYLALANINE
4-(tert-Butoxycarbonylamino)-NALPHA-9-fluorenylmethoxycarbonyl-L-phenylalan
4-(Boc-aMino)-N-FMoc-L-phenylalanine, 95%
4-[[(tert-Butoxy)carbonyl]amino]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-phenylalanine
FMoc-L-phe(Boc-4-AMino)-OH
(S)-2-(((9H-fluoren-9-yl)Methoxy)carbonylaMino)-3-(4-(tert-butoxycarbonylaMino)phenyl)propanoic acid
L-Phenylalanine,4-[[(1,1-diMethylethoxy)carbonyl]aMino]-N-[(9H-fluoren-9-ylMethoxy)carbonyl]-
Fmoc-Phe(Boc-4-NH2)-OH
(S)-Fmoc-2-amino-3-(4-Boc-aminophenyl)propionic acid
Fmoc-4-(Boc-amino)-L-phenylalanine≥ 99% (HPLC, Chiral purity)
Fmoc-p-(Boc-amino)-L-Phe-OH
4-[[(1,1-Dimethylethoxy)carbonyl]amino]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-phenylalanine
(2S)-3-(4-{[(tert-butoxy)carbonyl]amino}phenyl)-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)propanoic acid
(9H-Fluoren-9-yl)MethOxy]Carbonyl Phe(4-NHBoc)-OH
4-(Boc-amino)-N-Fmoc-L-phenylalanine,95%
Fmoc-p(NH-Boc)-L-Phe-OH USP/EP/BP
Fmoc-L-4-aminophe
M Fmoc-Phe(4-NHBoc)-OH
Fmoc-L-Phe(Boc-4-NH)-OH
(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[4-[(2-methylpropan-2-yl)oxycarbonylamino]phenyl]propanoic acid
174132-31-1
a-amino
Amino Acid Derivatives
Amino Acids
Phenylalanine analogs and other aromatic alpha amino acids





