9-ブロモアントラセン 化学特性,用途語,生産方法
外観
黄色~黄緑色, 結晶~結晶性粉末
溶解性
アセトンに溶け、水にほとんど溶けない。酢酸、二硫化炭素、ベンゼンに可溶。熱エタノールに微溶。
使用上の注意
不活性ガス封入
説明
9-bromoanthracene is a kind of bromine-derived anthracene. It is known to be able to reversibly photodimerize in a head-to tail fashion upon irradiation by long-wavelength ultraviolet light. The photodimers of 9-bromoanthracene are suitable to be used as alkyl halide initiators in the atom transfer radical polymerization (ATRP) reactions. It is also used as the intermediate for the preparation of the 9-substitued form of the polycyclic aromatic hydrocarbon (PAH) anthracene.
化学的特性
white to light yellow crystal powder. boiling point 190°C (0.16kPa, sublimation), relative density 1.409. soluble in acetic acid; carbon disulfide.
使用
9-Bromoanthracene acts as an intermediate in the preparation of 9-substituted derivative of the polycyclic aromatic hydrocarbon (PAH) anthracene.
主な応用
The Sonogashira reaction of 9-Bromoanthracene and ethynyltrimethylsilane gave not only the expected anticipated 9-(trimethylsilylethynyl)anthracene and 2-(trimethylsilyl)aceanthrylene and (E)-4-(9-anthracenyl)-1,3-bis(trimethylsilyl)-but-3-en-1-yne were also obtained in moderate yields. The mechanistic principle may be that the coupling of bromo(anthracenyl)bis(triphenylphosphine)palladium(II) can occur either directly or after coordination and migratory insertion of the free alkyne.
製造方法
Anthracene (5 g, 28.05 mmol) was dissolved in CHCl3. Then N-bromosuccinimide (NBS,4.99 g, 28.05 mmol) was added in batches away from light, and the reaction solution was continuously stirred for 12 h. The resulting mixture was stirred for another 30 min with appropriate water, and extracted with CH2Cl2. The CH2Cl2 solution was dried over anhydrous MgSO4. After removing CH2Cl2 solvent, the residue was recrystallized from anhydrous ethanol to give 4.78 g (66.3 %) of a green-yellow needle solid 9-Bromoanthracene. 1H NMR (500 MHz, CDCl3) δ 8.55 (d, J = 8.9 Hz, 2H), 8.48 (s, 1H), 8.03 (d, J = 8.4 Hz, 2H), 7.67 – 7.60 (m, 2H), 7.56 – 7.51 (m, 2H). EI-MS (m/z): Calculated for C14H9Br: 257.13. Found [M+ ]: 255.96.
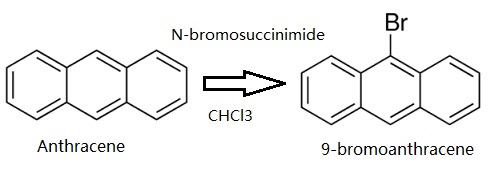
Synthesis of 9-bromoanthracene
純化方法
Crystallise 9-bromoanthracene from MeOH or EtOH followed by sublimation in vacuo. [Masnori et al. J Am Chem Soc 108 126 1986, Beilstein 5 IV 2295.]
参考文献
[1]Cohen, Nicole A., et al. Macromolecular Chemistry and Physics 210.3 ‐4 (2009): 263-268.
[2]Xu, Xiaoming, Wenzhe Lu, and Richard B. Cole. Analytical chemistry 68.23 (1996): 4244-4253.
[3]Dang, Hung, and Miguel A. Garcia-Garibay. Journal of the American Chemical Society 123.2 (2001): 355-356.
[4] Se Hun Kim. “Highly efficient deep-blue emitting organic light emitting diode based on the multifunctional fluorescent molecule comprising covalently bonded carbazole and anthracene moieties.” Journal of Materials Chemistry B 13 1 (2011): 9139–9148.
9-ブロモアントラセン 上流と下流の製品情報
原材料
準備製品