Radotinib Dihydrochloride 化学特性,用途語,生産方法
臨床応用
In January 2012, radotinib hydrochloride (marketed as Supect ®) obtained its approval from the KFDA
(Korea Food and Drug Administration) for the treatment of patients with Philadelphia chromosomepositive
chronic myeloid leukemia (CML) who have become resistant to existing drugs such as Gleevec,
Tasigna and Sprycel. Originally developed by IL-YANG pharmaceuticals of South Korea as an
orally second-generation tyrosine kinase inhibitor, the drug inhibits both Bcr-Abl fusion protein and the
platelet-derived growth factor receptor (PDGFR).
合成
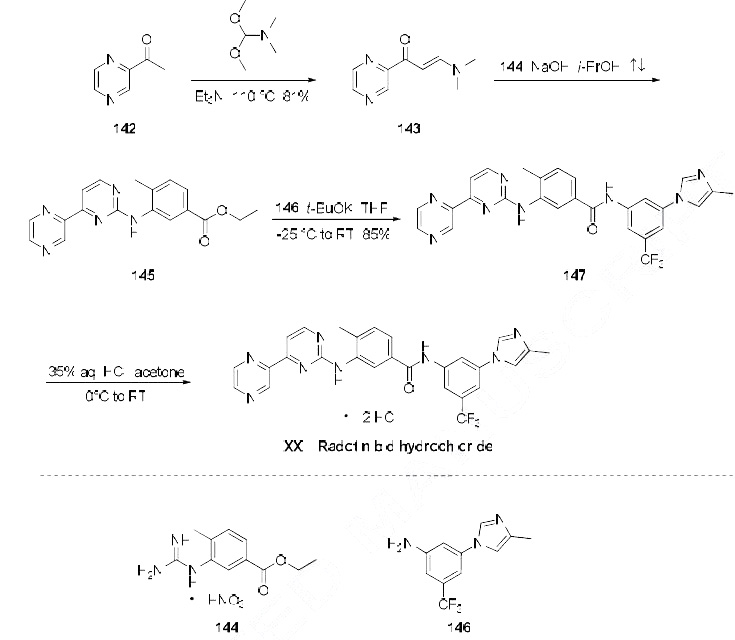
Because of the structural similarity of radotinib to
that of nilotinib (Tasigna ®), the process-scale synthetic route (which is depicted in the scheme) is
capable of furnishing both drugs.Claisen condensation of commerical 2-acetylpyrazine (142) with N,N-dimethylformamide
dimethylacetal gave rise to the enamino ketone 143 in 81% yield. Under basic conditions, vinylogous
amide 143 was coupled with commercial guanidine nitrate 144187 to produce aminopyridine 145.
Subsequent condensation with commercial aniline (146) by means of potassium t-butoxide in THF
constructed radotinib 147 in 85% yield as the free base, and this material could be converted to the
radotinib dihydrochloride (XXII) upon exposure to concentrated hydrochloric acid in chilled acetone.
Radotinib Dihydrochloride 上流と下流の製品情報
原材料
準備製品