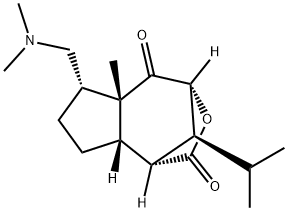
(1R,8aα,9S)-6β-[(Dimethylamino)methyl]-1,5a,6,7,8,8a-hexahydro-5aα-methyl-9-isopropyl-1,4α-methano-2H-cyclopent[d]oxepine-2,5(4H)-dione
|
|
(1R,8aα,9S)-6β-[(Dimethylamino)methyl]-1,5a,6,7,8,8a-hexahydro-5aα-methyl-9-isopropyl-1,4α-methano-2H-cyclopent[d]oxepine-2,5(4H)-dione 속성
- 녹는점
- 87-8°C
- 끓는 점
- 421.7±20.0 °C(Predicted)
- 밀도
- 1.089±0.06 g/cm3(Predicted)
- 산도 계수 (pKa)
- 9.26±0.28(Predicted)
안전
(1R,8aα,9S)-6β-[(Dimethylamino)methyl]-1,5a,6,7,8,8a-hexahydro-5aα-methyl-9-isopropyl-1,4α-methano-2H-cyclopent[d]oxepine-2,5(4H)-dione C화학적 특성, 용도, 생산
개요
Present in Dendrobiurn nobile, this alkaloid forms colourless crystals from MeOH. It yields a crystalline hydrochloride, m.p. 206-8°C and the methiodide, m.p. 275-6°C. Treatment of the free base with alcoholic KOH followed by HCI gives isonobiline hydrochloride, m.p. l83-5°C. LiAIH4 furnishes an amorphous tril whereas NaBH4 in tetrahydrofuran gives the amorphous hydroxynobiline which is converted into hydroxyisonobiline, m.p. 217 -9°C under the same conditions as given above for the alkaloid. Hofmann degradation of nobiline methiodide yields trimethylamine and deaminonobiline, m.p. l79-183°C. The structure given above for the alkaloid has been suggested on the basis of these chemical reactions and spectroscopic evidence.참고 문헌
Yamamura, Hirata., Tetrahedron Lett., 79 (1964)(1R,8aα,9S)-6β-[(Dimethylamino)methyl]-1,5a,6,7,8,8a-hexahydro-5aα-methyl-9-isopropyl-1,4α-methano-2H-cyclopent[d]oxepine-2,5(4H)-dione 준비 용품 및 원자재
원자재
준비 용품
(1R,8aα,9S)-6β-[(Dimethylamino)methyl]-1,5a,6,7,8,8a-hexahydro-5aα-methyl-9-isopropyl-1,4α-methano-2H-cyclopent[d]oxepine-2,5(4H)-dione 공급 업체
글로벌( 0)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|